Scroll to:
Preparation of recombinant SARS-CoV-2 nucleocapsid protein
https://doi.org/10.29326/2304-196X-2025-14-1-69-75
Abstract
Introduction. The new coronavirus infection (COVID-19) agent SARS-CoV-2 has become widespread in the world and has caused the pandemic that started in 2019. The virus is a zooanthroponotic infectious agent that causes infection in humans as well as in many mammal species. To date, SARS-CoV-2 has been reported both in domestic and in wild animals. Moreover, successful experimental infection of certain animal species was reported during the studies. There is also the evidence that infected animals can transmit the virus to other animals in natural settings through contactincluding virus transmission between animals of different species. Currently, some researchers fear that SARS-CoV-2 may spread to mammalian species in the wild that will become a natural reservoir responsible for this infection outbreaks in humans. Furthermore, the virus effect on potentially susceptible wild animal species, including endangered animal species, is currently not fully understood. Therefore, the infection spread in wild animals requires further study. This requires highly sensitive and specific diagnostic methods. Enzyme-linked immunosorbent assay (ELISA) using SARS-CoV-2 nucleocapsid protein as an antigen can be used for serological surveillance of the new coronavirus infection in animals. Recombinant protein used as an antigen is the most preferable because of its safety.
Objective. The study was aimed at preparing highly concentrated recombinant SARS-CoV-2 nucleocapsid protein and testing it for antigenic activity and specificity.
Materials and methods. The following was used for the study: SARS-CoV-2, pQE plasmid, Escherichia coli JM109 strain. The following was performed: reverse transcription and polymerase chain reaction, molecular cloning, recombinant protein synthesis, recombinant protein purification, indirect ELISA was used.
Results. Molecular cloning of SARS-CoV-2 N-gene was carried out using prokaryotic expression system. Escherichia coli clones producing 33 kDa recombinant SARSCoV-2 nucleocapsid protein were prepared. Optimal expression and purification conditions for highly concentrated antigen preparation were determined. It was shown that optimal inducer concentration was 0.5 mМ, optimal expression period was 4 hours. Urea at a concentration of 8 M as a denaturing agent and optimal imidazole concentration of 0.4 M in the elution buffer were selected based on the results of study of optimal conditions for recombinant antigen purification. Use of the optimal expression and purification procedure allowed us to prepare 1.5 mg of purified antigen from 100 mL of Escherichia coli culture. The recombinant protein demonstrated its high antigenic activity and specificity when tested with indirect ELISA.
Conclusion. Preparation of highly concentrated recombinant SARS-CoV-2 nucleocapsid protein enables its further use as an antigen for ELISA test system for detection of antibodies against SARS-CoV-2 nucleocapsid protein in animal sera.
For citations:
Yakovleva A.S., Kanshina A.V., Timina A.M. Preparation of recombinant SARS-CoV-2 nucleocapsid protein. Veterinary Science Today. 2025;14(1):69-75. https://doi.org/10.29326/2304-196X-2025-14-1-69-75
INTRODUCTION
The new coronavirus (SARS-CoV-2) caused a pandemic of acute respiratory infection that swept the world and led to the deaths of several million people [1]. The infected person can develop either asymptomatic disease or severe pneumonia resulting in death in case of total lung damage. SARS-CoV-2 is an enveloped single-stranded RNA-containing virus of the Coronaviridae family, Betacoronavirus genus. The virus virion has characteristic crown-like appearance with spike (S), membrane (M) and envelope (E) proteins located in a two-layer phospholipid envelope. The new coronavirus has 30 kb RNA genome encoding 16 non-structural proteins and 4 main structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N) [2].
Besides humans, SARS-CoV-2 is capable of infecting many mammalian species [2][3][4][5][6][7][8][9][10]. According to the World Organization for Animal Health (WOAH), the new coronavirus was detected in cats, dogs, rodents (hamsters), minks and ferrets, zoo animals (monkeys, tigers, lions, cougars, etc.), deer, foxes, horses. Since the beginning of the pandemic, 35 countries have reported SARS-CoV-2 in animals according to data given in the review prepared by the Rosselkhoznadzor Information Analysis Center [11].
Currently, it is found that the virus can be transmitted from one animal species to another, from humans to animals, and from animals to humans [12][13][14][15][16][17][18].
In May 2023, the World Health Organization has announced the end of the pandemic, but some groups of scientists consider this announcement premature. They suggest that the virus could pose a threat to public health for decades. Even if the virus circulation in the human population is completely eliminated, SARS-CoV-2 will pose a danger to human health and domestic and wild animal health due to hidden reservoirs in the wild [13][19][20].
Some animal species could play the role of a natural reservoir of this virus. Serological monitoring for the new coronavirus infection in domestic and wild animal populations is carried out in some countries to study this possibility. For example, in France, more than 5,600 serum samples from cats and dogs were tested for antibodies to SARS-CoV-2, and in China, more than 20,000 samples from these two animal species were tested. Seromonitoring of the new coronavirus infection in wild fauna is actively carried out in the USA. High seroprevalence has been found in raccoons, squirrels, red foxes, opossums, skunks, white-footed mice, and white-tailed deer [21][22][23].
Investigation of SARS-CoV-2 spread in wild and domestic animals requires appropriate diagnostic tools. In 2021, enzyme-linked immunosorbent assay (ELISA) test-system for detection of antibodies to SARS-CoV-2 in animal sera was developed at the Federal Centre for Animal Health [24]. Inactivated coronavirus is used as an antigen in this test-system. However, the virus cultivation is required for the preparation of such antigen that is associated with a high biological risk. Recombinant SARS-CoV-2 proteins produced in prokaryotic or eukaryotic expression systems can be a safer alternative to the antigen prepared from the native virus. Previously, the nucleocapsid protein was shown to be the most immunogenic SARS-CoV-2 protein having conserved amino acids [25][26].
The study was aimed at preparation of recombinant SARS-CoV-2 nucleocapsid protein for further use as an antigen for ELISA test-system.
MATERIALS AND METHODS
Virus. Clinical samples (nasal swabs) from the patient with confirmed COVID-19 were used for SARS-CoV-2 RNA extraction.
The viral RNA was recovered using GF/F glass fiber filters according to О. G. Gribanov et al. method [27].
Reverse transcription polymerase chain reaction (RT-PCR). SARS-CoV-2 N-gene was amplified with RT-PCR. The RT-PCR products were analyzed with agarose gel-electrophoresis at 50 мА using 1.5% agarose gel containing 0.001% ethidium bromide.
Molecular cloning of RT-PCR products was performed with common methods [28]. Also, pQE plasmid (plasmid QIAGEN, Netherlands), Escherichia coli JM109 strain (Promega, USA) were used.
Recombinant protein synthesis. Е. coli was cultivated in orbital shaker at 150 rpm and 37 °С. Isopropyl-β-D-1-thiogalactopyranoside (IPTG) was added to the cell culture that reached the logarithmic growth phase. Recombinant protein was analysed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Recombinant protein was purified with metal-chelate affinity chromatography using Ni-NTA-agarose (Thermo Fisher Scientific, США).
Indirect enzyme-linked immunosorbent assay (ELISA) was carried out using Tris buffered saline with Tween 20 (TBS-T) for plate washing. 1% milk in TBS-T was used for blocking nonspecific binding sites and for dilution of sera and secondary antibodies. Protein A conjugate (KPL Company, Italy) was used for tests; ABTS (2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) was used as a substrate for peroxidase conjugate.
RESULTS AND DISCUSSION
The fragment of N-gene of SARS-CoV-2 was amplified by RT-PCR using primers containing the BamHI and SalI restriction sites.
The pQE plasmid and the gene fragment were digested with restriction enzymes. A ligase mix containing the treated amplicon and plasmid vector were transformed into chemically competent cells of E. coli strain JM109. The cell pellet was spread on LB-agar containing 100 μg/mL of ampicillin. After transformation, the resulting colonies were screened and several E. coli clones expressing the recombinant SARS-CoV-2 nucleocapsid protein containing a polyhistidine tag at the N-terminus were selected. The molecular weight of the protein was 33 kDa that corresponded to the estimated data.
To increase the concentration of recombinant protein expressed in the selected E. coli clone, the optimal concentration of the inducer and the optimal expression period ensuring maximum protein accumulation in bacterial cells were determined.
Solutions of 0.1, 0.2, 0.5, and 1.0 mM IPTG were used to determine the optimal concentration of the inducer. The protein expression level was determined visually in 12% SDS-PAGE. The accumulation of recombinant protein reached a peak at an IPTG concentration of 0.5 mM and did not change when the inducer concentration was increased by twofold. The concentration of 0.5 mM was determined as optimal, and subsequently all expression runs were carried out using this inducer concentration.
To determine the optimal period of recombinant protein expression, E. coli cell lysate was examined 2, 4, and 18 hours after induction. The protein accumulation level was analyzed with 12% polyacrylamide gel electrophoresis. The maximum level of recombinant protein expression was observed 4 hours after adding of the inducer (Fig. 1). The protein amount was lower 18 hours after induction that may be accounted for its destruction during long-term E. coli cultivation. Thus, the optimal time for recombinant protein synthesis was considered to be 4 hours after induction.
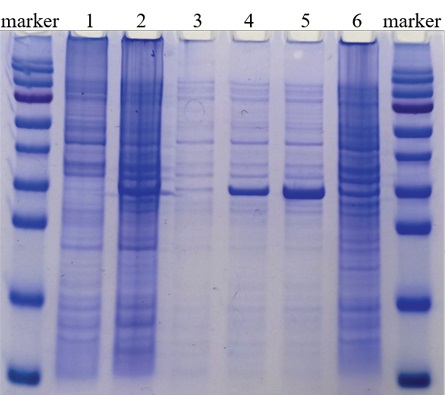
Fig. 1. Determination of optimal period
of recombinant SARS-CoV-2 nucleocapsid protein expression:
protein molecular weight marker
(170, 130, 95, 72, 55, 43, 34, 26, 17, 10 kDa);
1 – E. coli JM109 cell lysate;
2 – cell lysate of SARS-CoV-2 N-gene-containing E. coli clone, 18 hours after incubation;
3 – cell lysate of SARS-CoV-2 N-gene-containing E. coli clone, before induction;
4 – cell lysate of SARS-CoV-2 N-gene-containing E. coli clone, 2 hours after induction;
5 – cell lysate of SARS-CoV-2 N-gene-containing E. coli clone, 4 hours after induction;
6 – cell lysate of SARS-CoV-2 N-gene-containing E. coli clone, 18 hours after induction
The recombinant protein was purified with metal chelate chromatography. When cell lysate was prepared under native conditions, the most of the protein remained in the cellular debris, so further lysis was carried out under denaturing conditions.
To prepare purified protein at maximum concentration, studies were carried out to determine the optimal composition of lysis and elution buffers.
The following was included in the buffer composition: 8 M urea – for lysis buffer No. 1, and 6 M guanidine hydrochloride (Gu-HCl) – for lysis buffer No. 2. Under denaturing conditions, most of the protein was cleared from cellular debris by centrifugation. The protein yield was approximately the same when both 8 M urea and 6 M GuHCl were used (Fig. 2). It was decided to use 8 M urea as a denaturing agent.
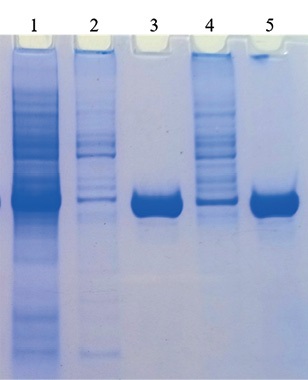
Fig. 2. Effect of lysing buffer composition
on purified recombinant SARS-CoV-2 nucleocapsid protein solubility and yield:
1 – cell lysate of recombinant E. coli clone, 4 hours after induction;
2 – sediment of recombinant protein-expressing cells
after treatment with lysis buffer No. 1 containing 8 M urea;
3 – purified recombinant protein when denaturing buffer containing 8 M urea was used;
4 – sediment of recombinant protein-expressing cells
after treatment with lysis buffer No. 2 containing 6 М Gu-HCl;
5 – purified recombinant protein when denaturing buffer containing 6 М Gu-HCl was used
Cell lysate was purified by metal chelate chromatography using Ni-NTA (nickel-nitrile acetate) agarose. Four buffer variants were used for N-protein elution: buffer A (8 M urea, 0.1 M Na2HPO4, 0.01 M tris-Cl, pH 4.0), buffer B (8 M urea, 0.1 M Na2HPO4, 0.01 M tris-Cl, 0.2 M imidazole, pH 8.0), buffer C (8 M urea, 0.1 M Na2HPO4, 0.01 M tris-Cl, 0.4 M imidazole, pH 8.0), buffer D (8 M urea, 0.1 M Na2HPO4, 0.01 M tris-Cl, 0.5 M imidazole, pH 8.0). The protein concentration in each eluate was assessed visually based on electrophoregram. The highest concentration of recombinant protein was found in the eluate derived using buffer C (Fig. 3). Thus, the maximum yield of purified N-protein was achieved by denaturation using buffer containing 8 M urea and elution using buffer containing 0.4 M imidazole.
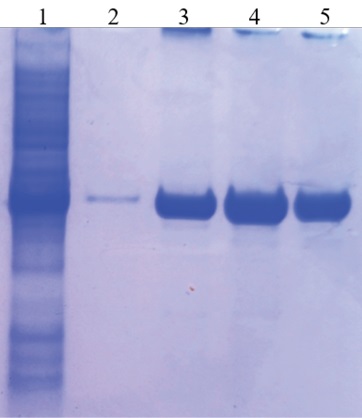
Fig. 3. Effect of elution buffer composition
on purified recombinant SARS-CoV-2 nucleocapsid protein yield:
1 – cell lysate of recombinant E. coli clone,4 hours after induction;
2 – purified recombinant protein when buffer A was used;
3 – purified recombinant protein when buffer B was used;
4 – purified recombinant protein when buffer C was used;
5 – purified recombinant protein when buffer D was used
During experiments for optimization of expression and purification conditions, the following scheme for recombinant SARS-CoV-2 nucleocapsid protein preparation was determined. One milliliters of recombinant E. coli clone cell suspension collected after overnight incubation was added to 0.1 L of LB medium supplemented with 100 μg/mL of ampicillin and incubated 37 °C at 150 rpm, until a density of OD600 = 0.5 was reached. For induction IPTG was added to a final concentration of 0.5 mM and the culture was incubated for another 4 hours at 37 °C and 150 rpm. The cell suspension was clarified at 5,000 rpm for 15 min. The pellet was resuspended with 5 mL of lysis buffer containing 8 M urea. The cellular debris was removed by centrifugation at 12,000 rpm for 5 min. The supernatant was used for metal chelate chromatography. The clarified lysate was mixed with 1 mL of sorbent (Ni-NTA agarose) for 15 min, then the resulting suspension was centrifuged at 12,000 rpm for 1 min. The pellet was washed twice with a lysis buffer. Elution was performed by adding 1 mL of elution buffer containing 0.4 M imidazole to the pellet, the pellet was stirred for 1 min, then centrifuged at 12,000 rpm for 1 min. The supernatant was tested for recombinant protein. The 12% polyacrylamide gel electrophoresis was performed to assess the degree of purification and the size of the resulting protein (Fig. 4). The protein concentration was measured by Bradford assay.
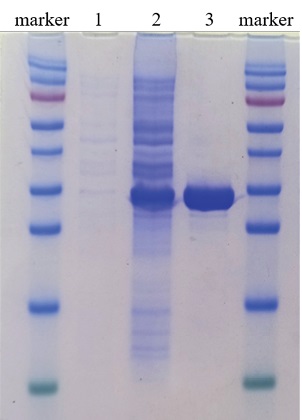
Fig. 4. Assessment of recombinant SARS-CoV-2 nucleocapsid protein size and purification level:
protein molecular weight marker
(170, 130, 95, 72, 55, 43, 34, 26, 17, 10 kDа);
1 – cell lysate of recombinant E. coli clone before induction;
2 – cell lysate of recombinant E. coli clone, 4 hours after induction;
3 – purified recombinant protein
Optimization of parameters of recombinant SARS-CoV-2 nucleocapsid protein expression and purification allowed for preparation of highly concentrated protein. The yield of purified protein from 100 mL of E. coli culture was 1.5 mg.
The recombinant SARS-CoV-2 nucleocapsid protein was tested for its antigenic activity in indirect ELISA using control rabbit sera. For this purpose, the recombinant protein diluted to 1:800 with carbonate-bicarbonate buffer was added to ELISA plate wells and the plate was incubated overnight. After blocking of non-specific binding sites and subsequent washing, control rabbit sera diluted from 1:20 to 1:2,560 were added to the plate wells. Sera from three rabbits immunized with the SARS-CoV-2 (+C) antigen were used as a positive control, and serum from non-immunized rabbit (–C) was used as a negative control. Sera were removed after 1 hour, the plate was washed, and protein A peroxidase conjugate was added. After another 1 hour, the plates were washed and ABTS substrate was added for reaction visualization. ELISA results are shown in the Table.
Table
Results of tests of recombinant SARS-CoV-2 nucleocapsid protein for its antigenic activity with indirect ELISA
|
Control serum dilution |
ELISA OD value of controls |
|||
|
–C |
+C No. 1 |
+C No. 2 |
+C No. 3 |
|
|
1:20 |
0.094 |
2.552 |
2.839 |
3.102 |
|
1:40 |
0.092 |
1.170 |
2.712 |
3.054 |
|
1:80 |
0.083 |
1.911 |
2.628 |
2.995 |
|
1:160 |
0.081 |
1.261 |
2.240 |
2.803 |
|
1:320 |
0.078 |
0.753 |
1.717 |
2.420 |
|
1:640 |
0.078 |
0.466 |
1.181 |
1.899 |
|
1:1,280 |
0.077 |
0.266 |
0.693 |
1.293 |
|
1:2,560 |
0.070 |
0.182 |
0.422 |
0.849 |
The studies showed that the recombinant nucleocapsid protein demonstrated high antigenic activity against positive control sera and absence of nonspecific binding with negative control serum.
CONCLUSION
The molecular cloning of the gene encoding the SARS-CoV-2 nucleocapsid protein was performed using a prokaryotic expression system.
E. coli clones expressing recombinant nucleocapsid protein were prepared.
The expression and purification conditions ensuring a high yield of purified antigen were determined.
Indirect ELISA test results have shown that prepared recombinant protein has high antigenic activity and can be used for detection of antibodies against SARS-CoV-2 in animal sera.
References
1. On-line coronavirus COVID-19 map. https://coronavirus-monitor.info (in Russ.)
2. Mahdy M. A. A., Younis W., Ewaida Z. An overview of SARS-CoV-2 and animal infection. Frontiersin Veterinary Science. 2020; 7:596391. https://doi.org/10.3389/fvets.2020.596391
3. Enserink M. Coronavirus rips through Dutch mink farms, triggering culls. Science. 2020; 368 (6496):1169. https://doi.org/10.1126/science.368.6496.1169
4. McAloose D., Laverac M., Wang L., Killian M. L., Caserta L. C., YuanF., et al. Frompeople toPanthera: Natural SARS-CoV-2 infectionintigers and lions at the Bronx Zoo. mBio. 2020; 11 (5):e02220-20. https://doi.org/10.1128/mbio.02220-20
5. Oreshkova N., Molenaar R. J., Vreman S., Harders F., Oude Munnink B. B., Hakze-van der Honing R. W., et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance. 2020; 25 (23):2001005. https://doi.org/10.2807/1560-7917.es.2020.25.23.2001005
6. Sailleau C., Dumarest M., Vanhomwegen J., Delaplace M., Caro V., Kwasiborski A., et al. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transboundary and Emerging Diseases. 2020; 67 (6): 2324–2328. https://doi.org/10.1111/tbed.13659
7. Freuling C. M., Breithaupt A., Müller T., Sehl J., Balkema-Buschmann A., Rissmann M., et al. Susceptibility of raccoon dogs for experimental SARS-CoV-2 infection. Emerging Infectious Diseases. 2020; 26 (12): 2982–2985. https://doi.org/10.3201/eid2612.203733
8. Sit T. H. C., Brackman C. J., Ip S. M., Tam K. W. S., Law P. Y. T., To E. M. W., et al. Infection of dogs with SARS-CoV-2. Nature. 2020; 586: 776–778. https://doi.org/10.1038/s41586-020-2334-5
9. Schlottau K., Rissmann M., Graaf A., Schön J., SehlJ., Wylezich C., et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. The Lancet Microbe. 2020; 1 (5): e218–e225. https://doi.org/10.1016/s2666-5247(20)30089-6
10. Hobbs E. С., Reid T. J. Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transboundary and Emerging Diseases. 2021; 68 (4): 1850–1867. https://doi.org/10.1111/tbed.13885
11. Makeeva Yu. Thirty-five WOAH member countries reported SARSCoV-2 in animals. Veterinary Medicine and Life. December 28, 2022. https:// vetandlife.ru/sobytiya/o-vyyavlenii-sars-cov-2-u-zhivotnyh-zayaviliv-vozzh-35-stran (in Russ.)
12. EFSA Panel on Animal Health and Welfare (AHAW), Nielsen S. S., Alvarez J., Bicout D. J., Calistri P., Canali E., et al. SARS-CoV-2 in animals: susceptibility of animal species, risk for animal and public health, monitoring, prevention and control. EFSA Journal. 2023; 21 (2):e07822. https://doi.org/10.2903/j.efsa.2023.7822
13. Fang R., Yang X., Guo Y., Peng B., Dong R., Li S., Xu S. SARS-CoV-2 infection in animals: Patterns, transmission routes, and drivers. EcoEnvironment & Health. 2024; 3 (1): 45–54. https://doi.org/10.1016/j.eehl.2023.09.004
14. Mastutik G., Rohman A., I’tishom R., Ruiz-Arrondo I., de BlasI. Experimental and natural infections ofsevere acute respiratory syndrome-related coronavirus 2 in pets and wild and farm animals. Veterinary World. 2022; 15 (3): 565–589. https://doi.org/10.14202/vetworld.2022.565-589
15. Cui S., LiuY., Zhao J., Peng X., LuG., ShiW., et al. An updated review on SARS-CoV-2 infection in animals. Viruses. 2022; 14 (7):1527. https://doi.org/10.3390/v14071527
16. Hammer A. S., Quaade M. L., Rasmussen T. B., Fonager J., Rasmussen M., Mundbjerg K., et al. SARS-CoV-2 transmission between mink (Neovison vison) and humans, Denmark. Emerging InfectiousDiseases. 2021; 27 (2): 547–551. https://doi.org/10.3201/eid2702.203794
17. Yen H.-L., Sit T. H. C., Brackman C. J., Chuk S. S. Y., Gu H., Tam K. W. S., et al. Transmission of SARS-CoV-2 delta variant (AY.127) from pet hamsters to humans, leading to onward human-to-human transmission: a case study. The Lancet. 2022; 399 (10329): 1070–1078. https://doi.org/10.1016/s0140-6736(22)00326-9
18. Cui X., Wang Y., Zhai J., Xue M., Zheng C., Yu L. Future trajectory of SARS-CoV-2: Constant spillover back and forth between humans and animals. Virus Research. 2023; 328:199075. https://doi.org/10.1016/j.virusres.2023.199075
19. Cheng K., Wu C., Gu S., LuY., Wu H., Li C. WHOdeclaresthe end of the COVID-19 global health emergency: lessons and recommendations from the perspective of ChatGPT/GPT-4. International Journal of Surgery. 2023; 109 (9): 2859–2862. https://doi.org/10.1097/js9.0000000000000521
20. Santini J. M., Edwards S. J. L. Host range of SARS-CoV-2 and implications for public health. The Lancet Microbe. 2020; 1 (4): e141–e142. https://doi.org/10.1016/s2666-5247(20)30069-0
21. Fritz M., Elguero E., Becquart P., de Fonclare D. de R., Garcia D., Beurlet S., et al. A large-scale serological survey in pets from October 2020 through June 2021 in France shows significantly higher exposure to SARSCoV-2 in cats. bioRxiv. December 26, 2022; preprint, Version 4. https://doi.org/10.1101/2022.12.23.521567
22. Wang A., Zhu X., Chen Y., Sun Y., Liu H., Ding P., et al. Serological survey of SARS-CoV-2 in companion animalsin China. Frontiersin Veterinary Science. 2022; 9:986619. https://doi.org/10.3389/fvets.2022.986619
23. Goldberg A. R., Langwig K. E., Brown K. L., Marano J. M., Rai P., King K. M., et al. Widespread exposure to SARS-CoV-2 in wildlife communities. Nature Communications. 2024; 15 (1):6210. https://doi.org/10.1038/s41467-024-49891-w.
24. Volkova M. A., Zinyakov N. G., Yaroslavtseva P. S., Chvala I. A., Galkina T. S., Andreychuk D. B. Development of the test kit for detection of SARSCoV-2 antibodies in sera of susceptible animals. Veterinary Science Today. 2021; (2): 97–102. https://doi.org/10.29326/2304-196X-2021-2-37-97-102
25. Burbelo P. D., Riedo F. X., Morishima C., Rawlings S., Smith D., Das S., et al. Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. The Journal of Infectious Diseases. 2020; 222 (2): 206–213. https://doi.org/10.1093/infdis/jiaa273
26. De Marco Verissimo C., O’Brien C., López Corrales J., Dorey A., Cwiklinski K., Lalor R., et al. Improved diagnosis of SARS-CoV-2 by using nucleoprotein and spike protein fragment 2 in quantitative dual ELISA tests. Epidemiology and Infection. 2021; 149:e140. https://doi.org/10.1017/S0950268821001308
27. Gribanov O. G., Shcherbakov A. V., Perevozchikova N. A., Gusev A. A. Purification of DNA fragments, plasmid DNA, and RNA using aerosil A-300 and GF/F (GF/C) filters. Biochemistry (Moscow). 1996; 61 (6): 764–768. https://elibrary.ru/ldmgwz
28. ManiatisT., Fritsch E. F., Sambrook J. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory; 1982. 545 p.
About the Authors
A. S. YakovlevaRussian Federation
Anastasia S. Yakovleva, Cand. Sci. (Biology), Senior Researcher, Reference Laboratory for Highly Dangerous Diseases
Yur’evets, Vladimir 600901
A. V. Kanshina
Russian Federation
Anzhelika V. Kanshina, Cand. Sci. (Veterinary Medicine), Senior Researcher, Reference Laboratory for Highly Dangerous Diseases
Yur’evets, Vladimir 600901
A. M. Timina
Russian Federation
Anna M. Timina, Cand. Sci. (Veterinary Medicine), Senior Researcher, Reference Laboratory for Highly Dangerous Diseases
Yur’evets, Vladimir 600901
Review
For citations:
Yakovleva A.S., Kanshina A.V., Timina A.M. Preparation of recombinant SARS-CoV-2 nucleocapsid protein. Veterinary Science Today. 2025;14(1):69-75. https://doi.org/10.29326/2304-196X-2025-14-1-69-75



































