Scroll to:
PCR-RFLP analysis of insecticide resistance to pyrethroids, organophosphates and carbamates in Musca domestica L.
https://doi.org/10.29326/2304-196X-2025-14-1-101-108
Abstract
Introduction. Zoophilic flies play a significant role in animal disease transmission, and insecticide resistance being a relevant veterinary issue globally is an obstacle to effective fly population control. Molecular methods are more commonly used to monitor and diagnose insecticide resistance in insect populations.
Objective. The study aims to assess distribution of the main mutations associated with resistance to pyrethroids, organophosphorus compounds and carbamates in three natural populations of Musca domestica L. collected in 2021–2023 in livestock facilities of the Tyumen Oblast.
Materials and methods. Genotyping of CYP, vssc and ace-2 genes was performed using polymerase chain reaction and restriction fragment length polymorphism.
Results. One mutation in the vssc gene (L1014F) associated with resistance to pyrethroids and two mutations in the ace-2 gene (G342A, G342V) conferring resistance to organophosphorus compounds and carbamates were found. The resistant allele L1014F was present in 40–70% of the tested insects of all three populations with 30–55% frequency. The G342A allele was found in 10 and 60% of insects from two populations with frequencies of 5 and 30%, respectively. The G342V allele was detected in 40% insects of only one population with a frequency of 25%.
Conclusion. The results obtained indicate the potential for conferring resistance to pyrethroids, organophosphorus compounds and carbamates in the studied populations of Musca domestica, which should be taken into account when selecting disinsectants for livestock-keeping facilities and protecting animals from insects. Further molecular tests of Musca domestica flies from the regions bordering the Tyumen Oblast will be useful for developing a strategy to contain spread of resistant alleles in local populations.
For citations:
Melnichuk A.D., Krestonoshina K.S., Kinareikina A.G., Maslakova K.Yu., Yangirova L.Ya., Silivanova E.A. PCR-RFLP analysis of insecticide resistance to pyrethroids, organophosphates and carbamates in Musca domestica L. Veterinary Science Today. 2025;14(1):101-108. https://doi.org/10.29326/2304-196X-2025-14-1-101-108
INTRODUCTION
Insects are a significant factor in the spread of various human and animal diseases [1][2], including synanthropic and zoophilic flies, in particular Musca domestica L. house fly (Diptera: Muscidae) [3,][4]. The ability of adult M. domestica to be a mechanical vector of such pathogens as helminth eggs, protozoa, viruses and bacteria, including antibiotic-resistant strains, has been demonstrated in a number of studies [4][5][6][7]. Thus, Mannheimia haemolytica, Pasteurella multocida and Histophilus somni causing bovine respiratory diseases were recovered from M. domestica collected at feedlots from animals suffering from bovine respiratory disease symptoms [5]. When homogenates prepared from house flies from US dairy and livestock farms were tested, tetracycline and florphenicol resistance genes with prevalence ranging from 5 to 95.8% were identified in recovered bacteria [6]. The ability of Newcastle disease virus to persist in an infectious dose in the gut of flies for four days after feeding with infected milk and for one day in chicken droppings has been shown under laboratory conditions [7], which increases the risk of disease spread via flies present in poultry farms. Given the veterinary importance of zoophilic flies, it is necessary to control their numbers.
Despite the great interest in pest control biological methods, the chemical method based on the use of synthetic insecticidal agents remains widely used. Synthetic pyrethroids, neonicotinoids, organophosphorus compounds (OPCs), and carbamates are most often used for protecting animals from insects and disinsecting livestock premises both in Russia and abroad [4][8]. M. domestica quite rapidly develop resistance against insecticides when used intensively: for example, more than 20-fold increase of resistance to permethrin [9] and alpha-cypermethrin [10] was revealed under laboratory conditions over 10–20 generations. According to a number of studies, resistance to pyrethroids (deltamethrin, permethrin, beta-cyfluthrin, cypermethrin) was observed in house fly field populations in China [11][12], Pakistan [9], Iran [13], USA [14], Saudi Arabia [10][15], the Moscow and Kaluga Oblasts of the Russian Federation [8]. In the Tyumen Oblast, tolerant and exceptionally highly pyrethroid-resistant field populations were also recorded [16][17]. OPC-resistant house fly populations were found, for instance, in China [12], Iran [18], and Saudi Arabia [15][19]. Insecticide resistance of M. domestica field populations makes it difficult to control their numbers.
The molecular target of pyrethroids is voltage-sensitive sodium channels (vssc), and the presence of mutations in the genes encoding this protein, i.e. knock-down resistance (kdr), is recognised as a marker of resistance to pyrethroids [14][20]. Of the five known alleles associated with target insensitivity and, consequently, pyrethroid resistance of insects, the kdr (L1014F) and kdr-his (L1014H) are the most frequently investigated [13][14][20]. Target insensitivity is often combined with another major mechanism of pyrethroid resistance, namely enhanced detoxification of insecticides via cytochrome P450-dependent monooxygenases (CYP). A confirmed molecular marker of this type of resistance is the presence of a 15-base pair (bp) insertion in the CYP6D1 gene [21][22]. Acetylcholinesterase (AChE), encoded by the ace gene, is a key enzyme of the cholinergic system and a major target of OPC and carbamate insecticides, which block the transmission of nerve impulses at cholinergic synapses. Resistance to OPC and carbamates may result from insensitivity of AChE due to mutations in the ace gene or due to mutations in the carboxylesterase gene, leading to an increase in the hydrolytic activity of the enzyme with respect to OPC [20][23]. M. domestica is known to have only one AChE-encoding gene, ace-2 [24], and six major mutations associated with resistance to OPC and carbamates have been described in detail: V260L, A316S, G342A, G342V, F407Y, and G445A [25][26][27].
Analysis of insecticide resistance in M. domestica field populations in Russia is traditionally carried out using toxicological methods [8][17][28], which allow establishing the presence of a stable phenotype and the level of resistance and do not explain the mechanisms underlying insecticide resistance [29]. The resistance mechanisms are defined and the potential for its formation is assessed using biochemical and molecular methods [30], and these steps are critical for rationalized selection of insecticidal agents and development of insecticide application schemes. As compared to traditional toxicological methods, molecular tests provide more complete information on the population structure, and the combination of toxicological and molecular methods allows objective assessment of the level of adaptation of the population to insecticide load [31]. Among molecular methods for detecting mutations associated with insecticide resistance, PCR-RFLP (polymerase chain reaction – restriction fragment length polymorphism) is used [32]. The PCR-RFLP method is cost-effective, easy to implement and requires only basic molecular genetic equipment; it is widely available and is a good alternative to sequencing.
The aim of the study was to test Musca domestica flies collected from three field populations in the Tyumen Oblast for the presence of mutations in CYP, vssc and ace-2 genes associated with resistance to pyrethroids, OPC and carbamates by PCR-RFLP.
MATERIALS AND METHODS
The study was aimed at M. domestica flies of three field populations: Nov (56.53700°, 65.24238°), Cha (56.781583°, 65.96014°), Nik (55.55352°, 70.62864°) collected in livestock facilities of the Tyumen Oblast in 2021–2023. The first generation (F1) was obtained from the collected insects of each population under insectarium conditions, 3–5 day old adult flies were frozen and stored at –80 °C before they were used for testing.
DNA was isolated from adult flies (5 females and males of each population) using alkaline lysis [33]. The amplification process was performed with GeneExplorer GE-96G (Bioer, China) using an individual primer pair for each gene. P1, P2, P3, P4 primers were used for genotyping mutations in the vssc gene, and AceF and AceR primers taken from the study of X. Qiu et al. [32] were used for the ace-2 gene. For genotyping of mutation in the CYP6D1 gene, the S35 and AS2 primers and restrictase were used according to F. D. Rinkevich et al. [34]. The amplification conditions were identical except for the temperature of primer annealing (Table 1): at 95 °C for 5 min, further at 95 °C for 20 s, at 62–53 °C for 30 s, at 72 °C for 30 s (5 cycles), at 95 °C for 20 s, at 60–51 °C for 30 s, at 72°C for 30 s (35 cycles), at 72 °C for 10 min. The PCR reaction mixture included: 1 µL of total DNA; 4 µL of 5X ScreenMix-HS PCR prepared mix (Eurogen, Russia); 0.3 µL of each primer (25 μM); 14.4 µL of purified sterile water (18.2 μS/cm). The restriction enzymes and test conditions are indicated in Table 1. Visualization of restriction results was performed through electrophoresis with 2% agarose gel containing ethidium bromide.
Table 1
PCR-RFLP assay conditions
|
Gene |
Primers (5’–3’) |
Annealing temperature, °C |
Amplicon length, bp |
Restrictase |
Mutation |
Restriction conditions |
|
vssc |
P1. GTGCTGTGCGGAGAGTGG P2. GAAGCCTCCATCCTGGGAG |
60 |
156 |
Sse9I |
L1014F |
3 h – 55 °C; 20 min – 65 °C |
|
P3. AGCTGTATACCCTTCTTCT P4. CGAAGTTGGACAAAAGCAAA |
51 |
220 |
Fat I |
L1014H |
||
|
CYP6D1 |
S35. AGCTGACGAAATTGATCAATCAGT AS2. CATTGGATCATTTTTCTCATC |
59 |
732–711 |
Hpy 188III |
CYP6D1v |
1 h – 37 °C; 20 min – 65 °C |
|
ace-2 |
AceF. CGGTGCATTTGGGTTTCTAC AceR. CGTAACCGCTAAGATCTGCTG |
57 |
609 |
Mh1 I |
G342 |
3 h – 37 °C; 20 min – 80 °C |
|
Aco I |
G342A |
3 h – 37 °C; 20 min – 65 °C |
RESULTS AND DISCUSSION
The prevalence and frequency of mutations associated with resistance to pyrethroids and OPCs have been investigated in M. domestica field populations in Denmark [35], Turkey [36], Iran [26][37], USA [14][34], Kazakhstan [22], United Arab Emirates (UAE) [38] and other countries. Regarding M. domestica populations in the Russian Federation, resistance to pyrethroids and other insecticides was previously assessed using mainly toxicological methods [8][17][28]. Data on molecular test results of the house fly field populations and the genetic potential for insecticide resistance in local populations of the Russian Federation have not been published in the open access.
Sse9I and Fat I restrictases are used for vssc genotyping with PCR-RFLP. The Sse9I restrictase cuts the amplicon into 2 fragments of 96 and 60 bp, respectively, in the presence of the L1014F mutation. The L1014H mutation is detected using the Fat I enzyme, which, in the presence of the mutation, cuts the 220 bp amplicon into fragments of 170 and 50 bp long, respectively [22]. Combining the both test results, we identified the following genotypes (Fig. 1): 1014 (L/L), 1014 (L/F), 1014 (F/F). The L1014F mutation was detected in 70% of the tested flies of the Nov and Cha populations and in 40% of the flies of the Nik population (Table 2).
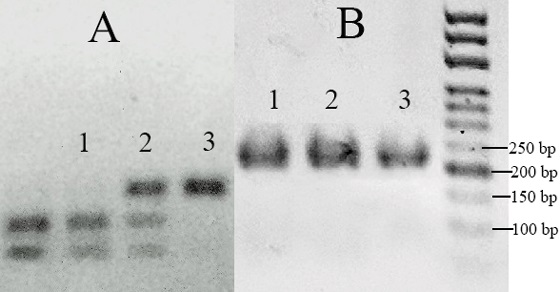
Fig. 1. Electrophoregram for PCR-RFLP amplification products of the vssc gene region: A – using Sse9I restrictase; B – using Fat I restrictase; 1 –1014 (F/F), 2 – 1014 (L/F), 3 – 1014 (L/L)
Table 2
Distribution of detected mutations associated with insecticide resistance in three populations of M. domestica in the Tyumen Oblast
|
Population |
Number of flies |
Proportion of flies with L1014F mutation, % |
Number of flies with the genotype |
Allele frequency, % |
Proportion of flies with mutation, % |
Number of flies with the genotype |
Allele frequency, % |
||||||
|
L/L |
L/F |
F/F |
F |
G342A |
G342V |
G/G |
G/A |
G/V |
A |
V |
|||
|
Nov |
10 |
70 |
3 |
3 |
4 |
55 |
0 |
0 |
10 |
0 |
0 |
0 |
0 |
|
Cha |
10 |
70 |
3 |
4 |
3 |
50 |
60 |
0 |
4 |
6 |
0 |
30 |
0 |
|
Nik |
10 |
40 |
6 |
2 |
2 |
30 |
10 |
40 |
5 |
1 |
4 |
5 |
25 |
Hpy 188III restrictase is used for CYP genotyping with PCR-RFLP. The resistant allele CYP6D1v1 is characterised by a 15 bp insertion that disrupts the recognition sequence of the Hpy 188III enzyme. As a result, after restriction, fragments of 432 and 279 bp will be characteristic of the wild-type genotype, and 732 bp will be characteristic of the genotype carrying the mutation [34]. No resistant allele of CYP6D1v1 was detected during the study, but Figure 2 shows that in some flies the 432 bp band is additionally cut by the Hpy 188III enzyme.
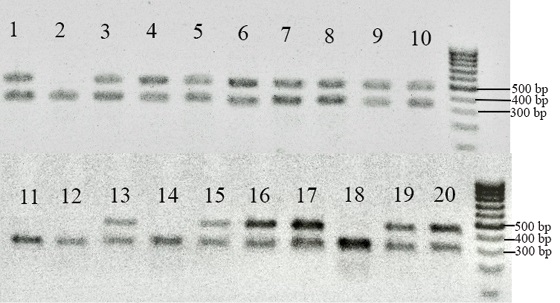
Fig. 2. Electrophoregram for PCR-RFLP amplification products of CYP6D1 gene region using Hpy 188III restrictase: 1–20 – different M. domestica species
PCR-RFLP assay of the ace-2 gene was performed using Mh1 I and Aco I enzymes. The Mh1 I restrictase has a restriction site (GGC) that is characteristic of the wild-type genotype, 342G. After restriction, the two fragments of 361 and 248 bp detected in the electrophoregram are indicative of a wild type genotype, and a 609 bp fragment is indicative of the G342A or G342V mutation. The Aco I restrictase identifies the G342A mutation and cuts the amplicon into 2 fragments of 361 and 248 bp long. Thus, combining the two assays allows the detection of 6 different genotypes [32]. In our study we managed to detect 3 different genotypes (Fig. 3). G342A or G342V mutations were found in the Nov population. In the Nik population, the proportion of flies with G342A and G342V mutations was 10 and 40%, respectively. In the Cha population, only G342A mutation was detected in 60% of flies (Table 2).
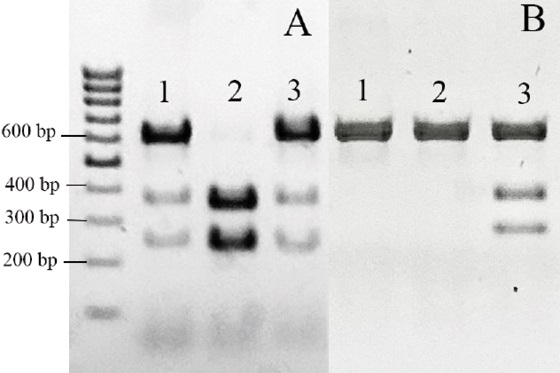
Fig. 3. Electrophoregram for PCR-RFLP amplification products of ace-2 gene region: A – using Mh1 I restrictase;
In total, 3 (L1014F, G342A, G342V) out of 5 tested mutations were identified using the PCR-RFLP. The distribution frequencies of the resistant alleles in the three populations are presented in Table 2. The kdr mutation (L1014F) was found in the hetero- and homozygous state in 7 out of 10 flies of the Nov and Cha populations and in 4 out of 10 flies of the Nik population. The kdr-his mutation (L1014H) was not detected in any of the three populations. Test results for field populations of M. domestica in Turkey showed that the frequency of kdr and kdr-his alleles was 8 and 20%, respectively [36]. A survey of six field populations of the house fly in Kazakhstan showed the presence of the kdr allele in one of the populations with a frequency of 5% and the kdr-his allele in another population with a frequency of 14.3% in the heterozygous state [22]. Interestingly, the L1014F mutation was not reported in the Iranian population of M. domestica, and the percentage of kdr-his polymorphism (L1014H) was low at 4.7% [37]. On the contrary, in the USA, the kdr (L1014F) mutation was present in all six studied populations of house flies found in poultry and livestock farms, and kdr-his (L1014H) mutation was present in five populations. The frequency of kdr-his and kdr alleles varied widely in the populations, ranging from 12.5–28.1% and 7.1–76.6%, respectively [14]. A recent paper reported the detection of the kdr allele in M. domestica flies from the United Arab Emirates with the frequencies ranging from 9.4 to 46.9% [38]. The frequency of the resistant kdr allele (30–55%) in house fly populations in the Tyumen Oblast is comparable to that of populations from the USA and the UAE.
According to literature data, the knockdown resistance was first reported in house flies in the 1950s as insensitivity of sodium channels to the action of dichlorodiphenyltrichloroethane (DDT). It was later found that such resistance was associated with a nucleotide substitution (cytosine for thymine) in the vssc gene, resulting in the replacement of leucine with phenylalanine at position 1014 (L1014F) of the sodium channel alpha subunit [39]. As a result, structural changes in the protein molecule occur, affecting the interaction of the insecticide with the target. This mutation also leads to the formation of resistance to pyrethroids, as they have a similar mechanism of action to DDT. The L1014F mutation, in addition to M. domestica, has been found in other two-winged insects (e.g., Culex and Anopheles mosquitoes, Haematobia fatheads), red cockroach (Blattella germanica), cat flea (Ctenocephalides felis), rat flea (Xenopsylla cheopis), triatomine bugs (e.g., Triatoma infestans), and other arthropods [39][40].
One of the sufficiently described mechanisms of resistance to pyrethroids in insects is the enhancement of detoxification mediated by cytochrome P450-dependent monooxygenases (CYP) [41]. This type of insecticide resistance in M. domestica is associated with increased expression of the CYP6D1 gene in the presence of 15 bp insertion (CYP6D1v1 allele) [34]. In the USA, the resistant CYP6D1v1 allele was detected with a frequency of > 75% in 5 studied populations of M. domestica [14]. According to V. Taşkın et al., the frequency of CYP6D1v1 in house fly population from Turkey was 39% [36]. In Kazakhstan, this allele was present in 3 out of 6 populations of M. domestica with a much lower frequency: 4.4–6.3% [22]. In our study, PCR-RFLP assay did not reveal an insertion characteristic of the resistant allele of CYP6D1v; however, a mutation described earlier for M. domestica laboratory culture was detected in flies from the Nov and Cha populations [42]. Freeman J. C. et al. rightly pointed out in their study that CYP6D1v1 is only partially responsible for the increased expression level of CYP6D1 [14]. Due to the high evolutionary plasticity of CYPs, their other representatives or other mutations not yet described may be involved in the formation of resistance to insecticides – in general, and pyrethroids – in particular, in local M. domestica populations.
Detection of a rather large percentage of flies with the kdr mutation among M. domestica of the three field populations under study is not surprising, since, according to the surveys, pyrethroids (mainly deltamethrin and cyfluthrin) had been used for premise disinsection and animal protection from annoying insects for several seasons in livestock farms where the flies were collected. The use of these insecticides in this case served as a selection factor that apparently allowed the kdr (L1014F) mutation to gain a foothold in the populations under study. It is believed that in the presence of the kdr (L1014F) mutation, a higher level of pyrethroid resistance is formed than in the presence of the kdr-his (L1014H) mutation [36][37]. In order to slow down the emergence of populations highly resistant to pyrethroids, it is advisable to replace pyrethroids with insecticides with a different mechanism of action (e.g., pyrroles, oxadiazines, insect growth regulators, etc.) in the studied livestock farms.
The higher Diptera have only one AChE-encoding gene and, accordingly, mutations providing resistance to OPCs and carbamates in this group of insects were found only in the ace-2 gene. Such mutations individually or in combination lead to amino acid substitutions close to the catalytic triad of the active centre of the enzyme, affecting the orientation of the amino acids of the triad and limiting the access and/or binding of bulk insecticides (enzyme inhibitors) in the substrate centre of the protein [25]. Six such mutations have been described in detail for M. domestica: V260L, A316S, G342A, G342V, F407Y and G445A [25][28]. In addition to M. domestica, resistance to OPCs and carbamates is known to be formed by a similar mechanism in other insect species, such as the green meat fly Lucilia cuprina [43], Drosophila melanogaster [44][45], and tephritid fruit flies Bactrocera oleae [46] and Bactrocera dorsalis [47]. In their study S. Başkurt et al. [48] indicated equivalent substitutions of amino acid residues in the AChE molecule for M. domestica and D. melanogaster. Literature data indicate that mutations underlying resistance of the house fly to OPCs and carbamates are widespread worldwide. Thus, resistant alleles G342A and G342V were found in flies of field populations of M. domestica of the USA, China, Iran, Kazakhstan [14][22][26][49]. In house fly populations from Kazakhstan, G342A and G342V resistant alleles were found with a frequency of 27–48 and 0–20%, respectively [22]. G342A and G342V mutations were detected in 30 and 40% of M. domestica flies from Iran, respectively [26]. In our study, the G342V resistant allele was only present in the Nik population (the mutation was present in 40% of flies) with a frequency of 25%, the G342A allele in the Nik (in 10% of flies) and Cha (in 60% of flies) populations with a frequency of 5 and 30%, respectively, and these mutations were not detected in the Nov population. It is assumed that the allele with the G342V mutation plays a more significant role in AChE insensitivity and the formation of a high level of resistance to certain insecticides compared to that with G342A mutation [14][25][49].
CONCLUSION
In this study, PCR-RFLP assay showed presence of the kdr allele (L1014F), responsible for resistance to pyrethroids, with a frequency of 30–55% and the G342A/V alleles associated with resistance to OPCs and carbamates, with a frequency of 5–30% in flies from three and two field populations of M. domestica in the Tyumen Oblast, respectively. The presented data indicate the potential for formation of resistance to pyrethroids, OPCs and carbamates in the studied populations. On the basis of the obtained results it is possible to recommend replacement of these insecticides during disinsection of livestock facilities with preparations from other groups in order to mitigate the spread of resistant alleles in local populations of M. domestica. Further molecular studies of insects from different regions of the country are required to assess more fully the situation regarding resistance to pyrethroids, OPCs and carbamates and the potential for its formation in M. domestica in Russia.
References
1. Domatskiy V. N., Fedorova O. A., Siben A. N. Epizootological and epidemiological place of sanguivorous dipterans in a climate of the Arctic (review). Russian Journal of Parasitology. 2018; 12 (4): 73–76. https://doi.org/10.31016/1998-8435-2018-12-4-73-76 (in Russ.)
2. Davlianidze T. A., Eremina O. Yu. Sanitary and epidemiological significance and resistance to insecticidesin the housefly Musca domestica. Plant Protection News. 2021; 104 (2): 72–86. https://doi.org/10.31993/2308-6459-2021-104-2-14984 (in Russ.)
3. Novak M. D., Engashev S. V., Mironenko A. V., Belova L. M., Engasheva E. S., Filimonov D. N. Dynamics of population size of blinds and zoophilic flies in the Central area of the Russian Federation. Veterinariya. 2020; (6): 28–32. https://doi.org/10.30896/0042-4846.2020.23.6.28-32 (in Russ.)
4. Geden C. J., Nayduch D., Scott J. G., Burgess E. R. IV, Gerry A. C., Kaufman P. E., et al. House fly (Diptera: Muscidae): biology, pest status, current management prospects, and research needs. Journal of Integrated Pest Management. 2021; 12 (1):39. https://doi.org/10.1093/jipm/pmaa021
5. Neupane S., Nayduch D., Zurek L. House flies (Musca domestica) pose a risk of carriage and transmission of bacterial pathogens associated with bovine respiratory disease (BRD). Insects. 2019; 10 (10):358. https://doi.org/10.3390/insects10100358
6. Neupane S., Talley J. L., TaylorD. B., NayduchD. Bacterial communities and prevalence of antibiotic resistance genes carried within house flies (Diptera: Muscidae) associated with beef and dairy cattle farms. Journal of Medical Entomology. 2023; 60 (6): 1388–1397. https://doi.org/10.1093/jme/tjad112
7. Chakrabarti S., King D. J., Cardona C. J., Gerry A. C. Persistence of exoticNewcastle disease virus (ENDV) in laboratory infected Musca domestica and Fannia canicularis. Avian Diseases. 2008; 52 (3): 375–379. https://doi.org/10.1637/8173-111407-Reg
8. Davlianidze T. A., EreminaO. Yu., OliferV. V. Resistance to insecticides of houseflyMusca domestica in the center ofthe European part of Russia. Plant Protection News. 2022; 105 (3): 114–121. https://doi.org/10.31993/2308-6459-2022-105-3-15346 (in Russ.)
9. Khan H. A. A. Characterization of permethrin resistance in a Musca domestica strain: resistance development, cross-resistance potential and realized heritability. Pest Management Science. 2019; 75 (11): 2969–2974. https://doi.org/10.1002/ps.5409
10. Abbas N., Hafez A. M. Alpha-cypermethrin resistance in Musca domestica: Resistance instability, realized heritability, risk assessment, and insecticide cross-resistance. Insects. 2023; 14 (3):233. https://doi.org/10.3390/insects14030233
11. Li Q., Huang J., Yuan J. Status and preliminary mechanism of resistance to insecticidesin a field strain of housefly (Musca domestica, L). Revista Brasileira de Entomologia. 2018; 62 (4): 311–314. https://doi.org/10.1016/j.rbe.2018.09.003
12. Wang J.-N., Hou J., Wu Y.-Y., Guo S., Liu Q.-M., Li T.-Q., Gong Z.-Y. Resistance of house fly, Musca domestica L. (Diptera: Muscidae), to five insecticides in Zhejiang Province, China: The situation in 2017. Canadian Journal of Infectious Diseases and Medical Microbiology. 2019; 2019 (1):4851914. https://doi.org/10.1155/2019/4851914
13. Ahmadi E., Khajehali J., Rameshgar F. Evaluation of resistance to permethrin, cypermethrin and deltamethrin in different populations of Musca domestica (L.), collected from the Iranian dairy cattle farms. Journal of Asia-Pacific Entomology. 2020; 23 (2): 277–284. https://doi.org/10.1016/j.aspen.2020.01.014
14. Freeman J. C., RossD. H., ScottJ. G. Insecticide resistance monitoring of house fly populationsfrom the United States. Pesticide Biochemistry and Physiology. 2019; 158: 61–68. https://doi.org/10.1016/j.pestbp.2019.04.006
15. Hafez A. M. First evaluation of field evolved resistance to commonly used insecticides in house fly populations from Saudi Arabian dairy farms. Insects. 2021; 12 (12):1120. https://doi.org/10.3390/insects12121120
16. Pavlov S. D., Pavlova R. P., Mavlyutov S. M. Orezistentnosti nasekomykh kompleksa gnus i komnatnoi mukhi k deistviyu sovremennykh insektitsidov = On resistance of gnat and housefly complex against modern insecticides. Entomologicheskie issledovaniya v Severnoi Azii: materialy VIIMezhregional’nogo soveshchaniya entomologov Sibiri iDal’nego Vostoka v ramkakh Sibirskoi zoologicheskoi konferentsii (Novosibirsk, 20–24 sentyabrya 2006 g.) = Entomological studies in Northern Asia: Proceedings of the VII Interregional Meeting of Entomologists of Siberia and the Far East within the framework of the Siberian Zoological Conference (Novosibirsk, September 20–24, 2006). Novosibirsk: Institute of Systematics and Ecology of Animals of Siberian Branch of Russian Academy of Sciences; 2006; 416–418. https://elibrary.ru/ttwhrz (in Russ.)
17. Levchenko M. A., Silivanova E. A., Hlyzova T. A., Bikinjaeva R. H., Metelitsa I. A. Susceptibility of Musca domestica (Diptera: Muscidae) field population to pyrethroid insecticides. Russian Journal “Problems of Veterinary Sanitation, Hygiene and Ecology”. 2017; (4): 71–75. https://elibrary.ru/ymeyas (in Russ.)
18. Ahmadi E., Khajehali J. Dichlorvos resistance in the house fly populations, Musca domestica, of Iranian cattle farms. Journal of Arthropod-Borne Diseases. 2020; 14 (4): 344–352. https://doi.org/10.18502/jad.v14i4.5271
19. Abobakr Y., Al-Hussein F. I., Bayoumi A. E., Alzabib A. A., Al-Sarar A. S. Organophosphate insecticidesresistance in field populations of house flies, Musca domestica L.: Levels of resistance and acetylcholinesterase activity. Insects. 2022; 13 (2):192. https://doi.org/10.3390/insects13020192
20. Eremina O. Yu., Lopatina Yu. V. Molecular genetic mechanisms of insecticide resistance in insects. Medical Parasitology and ParasiticDiseases. 2017; (4): 44–53. https://elibrary.ru/yurkfg (in Russ.)
21. Pan J., Yang C., Liu Y., Gao Q., Li M., Qiu X. Novel cytochrome P450 (CYP6D1) and voltage sensitive sodium channel (Vssc) alleles of the house fly (Musca domestica) and their roles in pyrethroid resistance. Pest Management Science. 2018; 74 (4): 978–986. https://doi.org/10.1002/ps.4798
22. Qu R., Zhu J., Li M., Jashenko R., Qiu X. Multiple genetic mutations related to insecticide resistance are detected in field Kazakhstani house flies (Muscidae: Diptera). Journal ofMedical Entomology. 2021; 58 (6): 2338–2348. https://doi.org/10.1093/jme/tjab110
23. Li Y., Farnsworth C. A., Coppin C. W., Teese M. G., Liu J.-W., Scott C., et al. Organophosphate and pyrethroid hydrolase activities of mutant Esterases from the cotton bollworm Helicoverpa armigera. PLoS ONE. 2013; 8 (10):e77685. https://doi.org/10.1371/journal.pone.0077685
24. KimY. H., Lee S. H. Which acetylcholinesterase functions asthe main catalytic enzyme in the ClassInsecta? Insect Biochemistry andMolecular Biology. 2013; 43 (1): 47–53. https://doi.org/10.1016/j.ibmb.2012.11.004
25. Walsh S. B., Dolden T. A., Moores G. D., Kristensen M., Lewis T., Devonshire A. L., Williamson M. S. Identification and characterization of mutations in housefly (Musca domestica) acetylcholinesterase involved in insecticide resistance. BiochemicalJournal. 2001; 359 (1): 175–181. https:// doi.org/10.1042/0264-6021:3590175
26. Adib D., Jafari A., Silivanova E., Basseri H., Gholizadeh S. Molecular analysis of acetylcholinesterase gene in field-collected populations of Musca domestica (Diptera: Muscidae) in Northwestern Iran. Journal of Insect Science. 2023; 23 (4):9. https://doi.org/10.1093/jisesa/iead054
27. Alzabib A. A., Al-Sarar A. S., Abobakr Y., Saleh A. A. Single and combined mutations of acetylcholinesterase gene giving resistance to pirimiphos-methyl inMusca domestica slaughterhouse populations. Insects. 2023; 14 (3):218. https://doi.org/10.3390/insects14030218
28. Levchenko M. A., Silivanova E. A., Shumilova P. A., Sennikova N. A. Insecticidal susceptibility and detoxification enzyme activities in Musca domestica L. (Diptera: Muscidae) of field populations. Russian Journal “Problems of Veterinary Sanitation, Hygiene and Ecology”. 2021; (4): 428–435. https://doi.org/10.36871/vet.san.hyg.ecol.202104008 (in Russ.)
29. R 4.2.3676-20 Laboratory methods of disinfectant tests and trialsto assesstheir effectiveness and safety: a study guide. Moscow: Federal Service for the Oversight of Consumer Protection and Welfare; 2020. https://docs.cntd.ru/document/573820733 (in Russ.)
30. World HealthOrganization. Manual for monitoring insecticide resistance in mosquito vectors and selecting appropriate interventions. Geneva: WorldHealthOrganization; 2022. 65 p. https://www.who.int/publications/i/item/9789240051089
31. Udalov M. B., Benkovskaya G. V. Population genetics of the Colorado potato beetle: from genotype to phenotype. Vavilov Journal ofGenetics and Breeding. 2011; 15 (1): 156–172. https://elibrary.ru/nypugv (in Russ.)
32. Qiu X., Pan J., Li M., Li Y. PCR-RFLP methods for detection of insecticide resistance-associated mutations in the house fly (Musca domestica). Pesticide Biochemistry and Physiology. 2012; 104 (3): 201–205. https://doi.org/10.1016/j.pestbp.2012.08.002
33. Bender W., Spierer P., Hogness D. S., Chambon P. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. Journal of Molecular Biology. 1983; 168 (1): 17–33. https://doi.org/10.1016/s0022-2836(83)80320-9
34. Rinkevich F. D., Zhang L., Hamm R. L., Brady S. G., Lazzaro B. P., Scott J. G. Frequencies of the pyrethroid resistance alleles of Vssc1 and CYP6D1 in house flies from the eastern United States. Insect Molecular Biology. 2006; 15 (2): 157–167. https://doi.org/10.1111/j.1365-2583.2006.00620.x
35. Huang J., Kristensen M., Qiao C. L., Jespersen J. B. Frequency of kdr gene in house fly field populations: correlation of pyrethroid resistance and kdr frequency. Journal of Economic Entomology. 2004; 97 (3): 1036–1041. https://doi.org/10.1093/jee/97.3.1036
36. TaşkınV., Başkurt S., Doğaç E., Taşkın B. G. Frequencies of pyrethroid resistance-associated mutations of Vssc1 and CYP6D1 in field populations of Musca domestica L. inTurkey. Journal ofVector Ecology. 2011; 36 (2): 239–247. https://doi.org/10.1111/j.1948-7134.2011.00164.x
37. Kamdar S., Farmani M., Akbarzadeh K., Jafari A., Gholizadeh S. Low frequency of knockdown resistance mutations in Musca domestica (Muscidae: Diptera) collected from Northwestern Iran. Journal of Medical Entomology. 2019; 56 (2): 501–505. https://doi.org/10.1093/jme/tjy177
38. Hamdan M., Kamalanathan T., Iqbal A., Gnanaprakasam A. R., Shajahan S., Alsadeq M. H., et al. kdr mutations and deltamethrin resistance in house flies in Abu Dhabi, UAE. Parasites & Vectors. 2024; 17 (1):47. https://doi.org/10.1186/s13071-024-06128-5
39. Rinkevich F. D., Du Y., Dong K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pesticide Biochemistry and Physiology. 2013; 106 (3): 93–100. https://doi.org/10.1016/j.pestbp.2013.02.007
40. Hutton S. M., Miarinjara A., Stone N. E., Raharimalala F. N., Raveloson A. O., Rakotobe Harimanana R., et al. Knockdown resistance mutations are common and widely distributed in Xenopsylla cheopis fleas that transmit plague in Madagascar. PLoS Neglected Tropical Diseases. 2023; 17 (8):e0011401. https://doi.org/10.1371/journal.pntd.0011401
41. Liu N., Li M., Gong Y., Liu F., Li T. Cytochrome P450s – Their expression, regulation, and role in insecticide resistance. Pesticide Biochemistry and Physiology. 2015; 120: 77–81. https://doi.org/10.1016/j. pestbp.2015.01.006
42. Krestonoshina K., Melnichuk A., Kinareikina A., Maslakova K., Yangirova L., Silivanova E. The P450-monooxygenase activity and CYP6D1 expression in the chlorfenapyr-resistant strain of Musca domestica L. Insects. 2024; 15 (6):461. https://doi.org/10.3390/insects15060461
43. Chen Z., Newcomb R., Forbes E., McKenzie J., Batterham P. The acetylcholinesterase gene and organophosphorus resistance in the Australian sheep blowfly, Lucilia cuprina. Insect Biochemistry and Molecular Biology. 2001; 31 (8): 805–816. https://doi.org/10.1016/s0965-1748(00)00186-7
44. Mutero A., Pralavorio M., Bride J. M., Fournier D. Resistance-associated point mutationsin insecticide-insensitive acetylcholinesterase. Proceedings of the National Academy of Sciences. 1994; 91 (13): 5922–5926. https://doi.org/10.1073/pnas.91.13.5922
45. Menozzi P., Shi M. A., Lougarre A., Tang Z. H., Fournier D. Mutations of acetylcholinesterase which confer insecticide resistance in Drosophila melanogaster populations. BMC Evolutionary Biology. 2004; 4:4. https://doi.org/10.1186/1471-2148-4-4
46. Pereira-Castro I., van Asch B., Trindade Rei F., Teixeira Da Costa L. Bactrocera oleae (Diptera: Tephritidae) organophosphate resistance alleles in Iberia: Recent expansion and variable frequencies. European Journal of Entomology. 2015; 112 (1): 20–26. https://doi.org/10.14411/eje.2015.019
47. Hsu J.-C., Haymer D. S., Wu W.-J., Feng H.-T. Mutations in the acetylcholinesterase gene of Bactrocera dorsalis associated with resistance to organophosphorus insecticides. Insect Biochemistry and Molecular Biology. 2006; 36 (5): 396–402. https://doi.org/10.1016/j.ibmb.2006.02.002
48. Başkurt S., Taşkın B. G., Doğaç E., Taşkın V. Polymorphism in the acetylcholinesterase gene of Musca domestica L. field populations in Turkey. Journal of Vector Ecology. 2011; 36 (2): 248–257. https://doi.org/10.1111/j.1948-7134.2011.00165.x
49. Yang X., Mou R., Liang Q., Cheng J., Wu Y., Tan W., Wu J. Frequency and polymorphism of acetylcholinesterase gene involved in the organophosphate resistance of Musca domestica in Guizhou Province, China. Archives of Insect Biochemistry and Physiology. 2023; 114 (3):e22045. https://doi.org/10.1002/arch.22045
About the Authors
A. D. MelnichukRussian Federation
Anastasia D. Melnichuk, Junior Researcher, Laboratory of Insect Molecular Biology and Biotechnology
2 Institutskaya str., Tyumen 625041
K. S. Krestonoshina
Russian Federation
Kseniya S. Krestonoshina, Head of Laboratory of Insect Molecular Biology and Biotechnology
2 Institutskaya str., Tyumen 625041
A. G. Kinareikina
Russian Federation
Anna G. Kinareikina, Postgraduate Student, Junior Researcher, Laboratory of Insect Molecular Biology and Biotechnology
2 Institutskaya str., Tyumen 625041
K. Yu. Maslakova
Russian Federation
Kseniya Yu. Maslakova, Junior Researcher, Laboratory of Insect Molecular Biology and Biotechnology
2 Institutskaya str., Tyumen 625041
L. Ya. Yangirova
Russian Federation
Liana Ya. Yangirova, Postgraduate Student, Junior Researcher, Laboratory of Insect Molecular Biology and Biotechnology
2 Institutskaya str., Tyumen 625041
E. A. Silivanova
Russian Federation
Elena A. Silivanova, Cand. Sci. (Biology), Leading Researcher, Laboratory of Insect Molecular Biology and Biotechnology
2 Institutskaya str., Tyumen 625041
Review
For citations:
Melnichuk A.D., Krestonoshina K.S., Kinareikina A.G., Maslakova K.Yu., Yangirova L.Ya., Silivanova E.A. PCR-RFLP analysis of insecticide resistance to pyrethroids, organophosphates and carbamates in Musca domestica L. Veterinary Science Today. 2025;14(1):101-108. https://doi.org/10.29326/2304-196X-2025-14-1-101-108
JATS XML



































