Scroll to:
Functional and metabolic activity of neutrophils in young cattle sensitized with a non-agglutinogenic strain of Brucella
https://doi.org/10.29326/2304-196X-2025-14-1-62-68
Abstract
Introduction. Brucellosis remains one of the most common highly dangerous zoonotic infections. Resistance to the pathogenic microorganisms of the genus Brucella depends on the appropriate cell-mediated immunity, which includes the activation of the bactericidal mechanisms of phagocytes. Despite the repeatedly proven role of neutrophils in the fight against many bacterial pathogens, the functions of these immunocompetent cells in the setting of brucellosis have long remained unstudied.
Objective. The study aimed to examine the functional and metabolic activity of neutrophils in young cattle sensitized with a non-agglutinogenic strain of Brucella.
Materials and methods. The functional and metabolic state of neutrophils in young cattle immunized against brucellosis with a vaccine produced from the non-agglutinogenic RB-51 strain of Brucella abortus was assessed on days 7, 14, 21, 28, 35 after immunization using nitroblue tetrazolium (NBT) test, as well as based on the level of the enzymatic activity of myeloperoxidase and the content of non-enzymatic cationic proteins. The measurements were made photometrically in the spontaneous and stimulated variants of the test, with subsequent calculation of stimulation coefficients. Disintegrated and corpuscular antigens prepared from Brucella vaccine strains with different antigen structures were used as reaction stimulants.
Results. It was found that the functional and metabolic status of neutrophils in young cattle immunized with the non-agglutinogenic strain of Brucella is characterized by increased neutrophil activity in the NBT test on days 7 and 35 of the experiment, by the absence of significant changes in the enzymatic activity of myeloperoxidase and a decrease in the content of non-enzymatic cationic proteins on days 7–14 after vaccination.
Conclusion. The most pronounced increase in stimulation coefficients was observed when using disintegrated Brucella antigens as a reaction stimulant. The highest stimulation coefficients were registered on day 28 after vaccination during the assessment of the oxygen-dependent metabolism of neutrophils with the NBT test and on day 14 during the assessment of the oxygen-independent metabolism.
For citations:
Manakova O.O., Yanchenko T.A., Vlasenko V.S. Functional and metabolic activity of neutrophils in young cattle sensitized with a non-agglutinogenic strain of Brucella. Veterinary Science Today. 2025;14(1):62-68. https://doi.org/10.29326/2304-196X-2025-14-1-62-68
INTRODUCTION
Despite the scientifically based system of brucellosis control measures in place in animal farming, bovine brucellosis remains endemic in most territories of the Russian Federation and poses a risk to livestock farms [1][2].
One of the main components of the said system is now specific prevention [3][4][5][6] mainly aimed at the reproduction of asymptomatic or latent infection in farm animals in combination with non-sterile immunity turning into post-infection sterile one [7][8][9].
The resistance of a macroorganism to the pathogenic microorganisms of the genus Brucella at the first stages of infectious process development depends on the activity of cellular protection factors, namely the activation of the bactericidal mechanisms of phagocytes [10][11][12]. Polymorphonuclear neutrophils are the main phagocytic cells responsible for protection against brucellosis. The functional and metabolic status of neutrophils determines the severity of the inflammatory reaction that develops in response to the entry of infectious pathogens into the body [13][14][15]. The bactericidal properties of neutrophils are provided by hydrolytic enzymes, cationic proteins and reactive oxygen species [16][17][18].
The examination of the enzymatic and non-enzymatic systems of neutrophils makes it possible to detect changes in the body at the early stages of infectious process development, prior to the occurrence of more profound changes in the organs and systems, which are detected with conventional test methods. Scientific literature describes the specific features of neutrophil system functioning revealed by tests in laboratory and other animals [19][20][21].
Cell-mediated immunity to brucellosis in food producing animals is an issue of particular scientific interest today. In vitro tests for cellular response to stimulation with Brucella antigens prepared at the Omsk Agrarian Scientific Center can be considered an informative and objective approach to analyzing the immunological restructuring of the body at the early post-vaccination stages, which is very important when evaluating the effectiveness of immunobiological products.
The study aimed to examine the functional and metabolic activity of neutrophils in young cattle sensitized with a non-agglutinogenic strain of Brucella.
MATERIALS AND METHODS
The work was performed at the Department of Veterinary Medicine of the Omsk Agrarian Scientific Center.
The test material was heparinized bovine peripheral blood. Sampling was carried out before vaccination and on days 7, 14, 21, 28, 35 after vaccination.
Bacterial strains. The antigens were prepared using the following Brucella strains from the bioresource collection of the Department of Veterinary Medicine of the Omsk Agrarian Scientific Center: Brucella abortus 16/4 in the stable R-form and Brucella abortus 19 in the S-form.
Young animals were immunized with bovine brucellosis vaccine based on the non-agglutinogenic RB-51 strain of B. abortus (the USA).
Animals. The experiment was carried out in 4–5-month-old Red Steppe heifers (n = 50). The animals were loose housed and received a balanced diet.
The antigens were prepared at the scientific laboratory using the modified methods of N. P. Ivanov [22].
CS is a corpuscular antigen prepared from B. abortus 19 strain.
CR is a corpuscular antigen prepared from B. abortus 16/4 strain.
DS is a disintegrated antigen prepared from B. abortus 19 strain by ultrasonic disintegration.
DR is a disintegrated antigen prepared from B. abortus 16/4 strain by ultrasonic disintegration.
The functional and metabolic state of neutrophils was assessed with nitroblue tetrazolium (NBT) test using a modified method [23], as well as based on the content of cationic proteins and myeloperoxidase using a modified method described by N. M. Khitrik [24]. The measurements were made photometrically in the spontaneous (without antigen treatment) and stimulated (with antigen treatment) variants of the test. The test results were read using a Fluorofot STD-Less-486-M multichannel immunochemistry analyzer (Russia) and expressed in relative optical density units, with subsequent calculation of the stimulation coefficient according to the following formula:
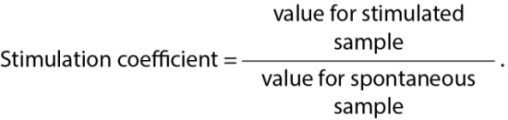
The disintegrated (DR and DS) and corpuscular (CR and CS) Brucella antigens were used as reaction stimulants.
The mathematical processing of the obtained numerical data was carried out using the standard methods of variation statistics involving the determination of arithmetic mean (M) values and the calculation of arithmetic mean errors (m). Student’s t-test was used to assess the significance of differences (p). We also applied the normalized deviation method involving automatic determination with a special computer software [25] using the following formula:
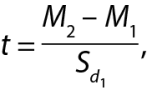
where t is the normalized deviation; M is the mean for the test (M2) and control (M1) groups; Sd is the standard deviation for the control group.
RESULTS AND DISCUSSION
The study was conducted on a brucellosis-free commercial farm where bovine brucellosis vaccine based on the non-agglutinogenic B. abortus RB-51 strain is used on a regular basis.
The initial stage of the study included the assessment of the functional and metabolic state of neutrophils with NBT test, tests for the enzymatic activity of myeloperoxidase and non-enzymatic cationic protein content carried out at different time points after the sensitization of the animals with the non-agglutinogenic strain of Brucella.
It was found that after an increase by day 7 from the start of the experiment, the spontaneous tetrazolium activity of neutrophils showed a slight downward trend and reached a minimum by day 28, then it increased again, but did not reach a significant difference as compared with the baseline values.
When the corpuscular (СS, CR) and disintegrated (DS, DR) antigens were added to the phagocyte cell suspension, the increased generation of oxygen radicals in neutrophil granulocytes was also observed by day 7; then it returned to the initial level (that before the administration of the vaccine) on days 14–28 from the start of the experiment depending on the antigen used. It should be noted that on day 35, there was again an increase in the induced NBT activity. In particular, a statistically significant 2.1-fold (p < 0.05) and 1.9-fold (p < 0.05) increase was registered after stimulation with the CS and DS antigens, respectively, as compared with the relevant values before vaccination.
From day 14 after vaccination, an increase in the NBT stimulation coefficient was observed when using the corpuscular CS, CR and disintegrated DS antigens as inducers, whereas the stimulating effect of the DR antigen was observed starting from day 21. It should also be noted that the stimulation coefficient reached its maximum values on day 28 from the start of the experiment, especially when the phagocytes interacted with the disintegrated DS and DR antigens (the coefficient increased 1.6- and 2.3-fold, respectively, as compared with the values before sensitization with Brucella). Subsequently, a decrease in the NBT stimulation coefficients was observed; however, the coefficient remained at the same level when the CS antigen was used (Table 1).
Table 1
Stimulation coefficient dynamics in tests for tetrazolium activity of neutrophil granulocytes in young cattle at different time points after vaccination, M ± m
|
Antigen |
Days after vaccination |
|||||
|
Before vaccination |
7 |
14 |
21 |
28 |
35 |
|
|
CS |
0.63 ± 0.17 |
0.65 ± 0.02 |
1.00 ± 0.28 |
1.05 ± 0.06 |
1.00 ± 0.08 |
1.03 ± 0.24 |
|
CR |
0.72 ± 0.31 |
0.75 ± 0.13 |
0.89 ± 0.10 |
0.85 ± 0.10 |
1.04 ± 0.14 |
0.80 ± 0.11 |
|
DS |
0.82 ± 0.44 |
0.71 ± 0.09 |
0.85 ± 0.22 |
0.87 ± 0.09 |
1.33 ± 0.19 |
0.80 ± 0.16 |
|
DR |
0.78 ± 0.25 |
0.70 ± 0.07 |
0.72 ± 0.14 |
1.18 ± 0.33 |
1.80 ± 0.32 |
0.73 ± 0.13 |
The data from the tests confirmed our previous findings. In particular, the highest stimulation coefficients in the tests for the tetrazolium activity of neutrophils in guinea pigs immunized with a non-agglutinogenic strain of Brucella had been observed on day 28 after immunization [26].
The spontaneous and stimulated enzymatic activity of myeloperoxidase, which also characterizes the oxygen-production ability of neutrophils, did not show any statistically significant changes during the dynamic tests. The stimulation coefficients reached their maximum values on day 21 from the start of the experiment, except when the disintegrated DS antigen was added to the blood samples (Table 2).
Table 2
Stimulation coefficient dynamics in tests for enzymatic activity of myeloperoxidase of neutrophil granulocytes in young cattle at different time points after vaccination, M ± m
|
Antigen |
Days after vaccination |
|||||
|
Before vaccination |
7 |
14 |
21 |
28 |
35 |
|
|
CS |
0.87 ± 0.13 |
0.99 ± 0.02 |
0.99 ± 0.01 |
1.02 ± 0.01 |
0.99 ± 0.01 |
1.00 ± 0.01 |
|
CR |
1.00 ± 0.02 |
0.99 ± 0.02 |
0.97 ± 0.01 |
1.03 ± 0.01 |
0.95 ± 0.01 |
0.97 ± 0.01 |
|
DS |
1.01 ± 0.03 |
1.00 ± 0.01 |
1.00 ± 0.004 |
0.98 ± 0.02 |
0.85 ± 0.10 |
0.99 ± 0.01 |
|
DR |
0.96 ± 0.02 |
0.98 ± 0.02 |
0.97 ± 0.01 |
1.03 ± 0.01 |
0.94 ± 0.01 |
0.96 ± 0.01 |
Neutrophil cationic proteins are another indicator, which, unlike the previous two, characterizes the anaerobic metabolism of phagocytes. The dynamic tests revealed certain specific features of its changes that were not observed during the tests for the tetrazolium and enzymatic activity of neutrophils. In particular, the spontaneous activity of antimicrobial peptides (2.15 ± 0.48) at the beginning of the experiment) reached a minimum (1.03 ± 0.03) on day 14 after the sensitization of the animals with Brucella and then, after a short-term slight increase (up to 1.23 ± 0.19) on day 28, decreased again (to 1.07 ± 0.11) on day 35.
When the blood samples were treated with the corpuscular antigen prepared from the S-strain, the activity of cationic proteins decreased 1.3-fold (p < 0.05) by day 14 from the start of the experiment, then 1.78-fold (p < 0.05) on day 28 and 1.67-fold (p < 0.05) on day 35 as compared with the baseline values. When the antigen prepared from Brucella R-strain was used, a significant decrease in the oxygen-independent metabolism of neutrophils was registered from day 21 after the administration of the non-agglutinogenic Brucella strain to young cattle.
When the disintegrated DS- and DR-antigens were used, a decrease in the activity of neutrophil cationic proteins was observed at a later time point. In particular, the antimicrobial activity of phagocytes decreased 1.73-fold (p < 0.05) and 2.24-fold (p < 0.05) on day 28 from the start of the experiment and 2.46-fold (p < 0.05) and 3.4-fold (p < 0.05) on day 35, respectively, as compared with the initial values.
On day 14 after the sensitization of the animals with Brucella, the stimulation coefficient reached its maximum value, with the most pronounced 1.54-fold (p < 0.05) and 1.68-fold (p < 0.05) increase, as compared with the baseline values, having been observed when using the disintegrated DS and DR antigens, respectively. At the subsequent time points of the study, the coefficient decreased (Table 3).
Table 3
Stimulation coefficient dynamics in tests for non-enzymatic cationic proteins of neutrophils in young cattle at different time points after vaccination, M ± m
|
Antigen |
Days after vaccination |
|||||
|
Before vaccination |
7 |
14 |
21 |
28 |
35 |
|
|
CS |
0.89 ± 0.18 |
1.22 ± 0.21 |
1.30 ± 0.10 |
1.49 ± 0.31 |
0.86 ± 0.12 |
0.96 ± 0.13 |
|
CR |
1.24 ± 0.14 |
1.25 ± 0.27 |
1.80 ± 0.26 |
1.03 ± 0.03a |
1.10 ± 0.26 |
1.38 ± 0.16 |
|
DS |
1.43 ± 0.18 |
1.45 ± 0.35 |
2.20 ± 0.19a |
1.41 ± 0.24 |
1.40 ± 0.20 |
1.16 ± 0.19 |
|
DR |
1.28 ± 0.18 |
1.31 ± 0.28 |
2.15 ± 0.11a |
1.52 ± 0.29 |
0.96 ± 0.12 |
0.74 ± 0.06a |
|
a p < 0.05. |
||||||
The stimulation coefficient dynamics in the tests for non-enzymatic cationic proteins of neutrophils in young cattle confirmed our previous findings from the experiment in guinea pigs immunized with a non-agglutinogenic strain of Brucella, during which the most pronounced activity of non-enzymatic cationic proteins of neutrophils was observed on day 14 after the administration of the immunobiological [27].
At the next stage of the study, a special computer software was applied to statistically process the stimulation coefficients of the NBT test, myeloperoxidase and cationic proteins, using the normalized deviation method to determine the degree of their transformation as compared with the mean values at different time points of the experiment. When the deviation from the mean exceeded +1.0 sigma, the difference was considered statistically significant, which was indicative of a pronounced specific sensitization of neutrophils to Brucella. The results are presented in Table 4.
Table 4
Determination of specific sensitization of neutrophils to Brucella by analysis of stimulation coefficients of NBT test, myeloperoxidase and cationic proteins
|
Antigen |
Days after vaccination |
||||
|
7 |
14 |
21 |
28 |
35 |
|
|
NBT test |
|||||
|
CS |
+0.05 |
+1.21 |
+1.39 |
+1.21 |
+1.30 |
|
CR |
+0.52 |
+0.30 |
+0.24 |
+0.60 |
+0.13 |
|
DS |
–0.14 |
+0.41 |
+0.61 |
+0.67 |
–0.20 |
|
DR |
–0.19 |
–0.14 |
+0.93 |
+2.38 |
–0.10 |
|
myeloperoxidase |
|||||
|
CS |
+0.50 |
+0.53 |
+0.67 |
+0.50 |
+0.54 |
|
CR |
–0.30 |
–0.72 |
+0.96 |
–1.47 |
–0.90 |
|
DS |
–0.17 |
–0.29 |
–0.53 |
–2.78 |
–0.44 |
|
DR |
+0.40 |
+0.22 |
+1.69 |
–0.66 |
–0.07 |
|
cationic proteins |
|||||
|
CS |
+1.06 |
+1.32 |
+1.93 |
–0.10 |
+0.21 |
|
CR |
+0.05 |
+2.23 |
–1.47 |
–0.56 |
+0.55 |
|
DS |
+0.06 |
+2.42 |
–0.07 |
–0.09 |
–0.85 |
|
DR |
+0.09 |
+2.76 |
+0.76 |
–1.01 |
–1.69 |
The data show that in the NBT test, a pronounced specific sensitization was registered on days 14–35 from the start of the experiment when using the corpuscular CS antigen and on day 28 when using the disintegrated DR antigen as a stimulant, whereas in the tests for the enzymatic activity of myeloperoxidase, it was registered only on day 21 when using the disintegrated DR antigen as an inducer (t = +1.69).
In the tests for cationic proteins, which perform their function under anaerobic conditions, specific sensitization was registered at earlier time points as compared with the indicators of oxygen-dependent metabolism. In particular, when the CS antigen was used, a deviation from the mean that exceeded +1.0 sigma was observed from day 7 to day 21, whereas when the CR-, DS- and DR-antigens were used, it was observed only on day 14. It should be noted that the disintegrated antigens induced a more pronounced specific sensitization (from +2.42 and above).
CONCLUSION
The results of the tests performed show that the functional and metabolic activity of neutrophils in young cattle immunized with B. abortus RB-51 strain is characterized by a 1.1–2.4-fold increase in the spontaneous and stimulated tetrazolium activity of neutrophils on days 7 and 35 after vaccination irrespective of the antigen used, a 1.2–2.1-fold decrease in the concentration of cationic proteins from days 7–14 from the start of the experiment and the absence of any pronounced changes in the content of myeloperoxidase.
A pronounced (1.5–2.3-fold) increase in the stimulation coefficients was observed when the disintegrated antigens were used. Based on the results of the mathematical processing involving the use of the normalized deviation method, the highest stimulation coefficients were observed during the assessment of the aerobic metabolism of neutrophils (NBT test) on day 28 after the inoculation of the vaccine strain when using the DR antigen (t = +2.38) and during the assessment of anaerobic metabolism (cationic proteins) on day 14 when using the DR and DS antigens (t = +2.42 and +2.76, respectively), which was indicative of a pronounced specific sensitization of neutrophils to Brucella.
References
1. Arakelyan P. K., Dimova A. S., Dimov S. K., Rudenko A. V., Yanchenko T. A., Orobets V. A. Ecological bases of the epizootic process of brucellosis and its control in small ruminants. IOP Conference Series: Earth and Environmental Science. 2021; 723:042015. https://doi.org/10.1088/1755-1315/723/4/042015
2. Gordienko L. N., Novikov A. N., Kulikova E. V. The dynamics of development the epizootic process in the new brucellosis focus, which was emerged against the background longtime welfare. Veterinariya. 2023; (6): 16–19. https://doi.org/10.30896/0042-4846.2023.26.6.16-19 (in Russ.)
3. Elrashedy A., Gaafar M., Mousa W., Nayel M., Salama A., Zaghawa A., et al. Immune response and recent advances in diagnosis and control of brucellosis. German Journal of Veterinary Research. 2022; 2 (1): 10–24. https://doi.org/10.51585/gjvr.2022.1.0033
4. Arakelyan P. K., Tregubov A. N., Vergun A. A., Ilyin E. N., Dimova A. S., Dimov S. K., Yanchenko T. A. The role of specific prevention in the control of the epizootic process of brucellosis cattle. Veterinariya. 2021; (11): 28–32. https://doi.org/10.30896/0042-4846.2021.24.11.28-32 (in Russ.)
5. Arakelyan P. K., Tregubov A. N., Vergun A. A., Ilin E. N., Yanchenko T. A., DimovaA. S., et al. Antiepizootic effectiveness of conjunctival immunization of cattle with a vaccine from the B. abortus 19 strain in brucellosis. Veterinariya. 2020; (10): 9–12. https://doi.org/10.30896/0042-4846.2020.23.10.09-12 (in Russ.)
6. Heidary M., Dashtbin S., Ghanavati R., Mahdizade Ari M., Bostanghadiri N., Darbandi A., et al. Evaluation of brucellosis vaccines: A comprehensive review. Frontiers in Veterinary Science. 2022; 9:925773. https://doi.org/10.3389/fvets.2022.925773
7. Gheibi A., Khanahmad H., Kashfi K., Sarmadi M., Khorramizadeh M. R. Development of new generation of vaccines for Brucella abortus. Heliyon. 2018; 4 (12):e01079. https://doi.org/10.1016/j.heliyon.2018.e01079
8. Simpson G.J.G., Marcotty T., Rouille E., Chilundo A., Letteson J.-J., Godfroid J. Immunological response to Brucella abortus strain 19 vaccination of cattle in a communal area in South Africa. Journal of the South African Veterinary Association. 2018; 89:a1527. https://doi.org/10.4102/jsava.v89i0.1527
9. Senevirathne A., Hewawaduge C., Lee J. H. Attenuated Salmonella secreting Brucella protective antigens confer dual-faceted protection against brucellosis and salmonellosis in a mouse model. Veterinary Immunology and Immunopathology. 2019; 209: 31–36. https://doi.org/10.1016/j.vetimm.2019.02.001
10. Zimmermann P., Curtis N. Factors that influence the immune response to vaccination. ClinicalMicrobiology Reviews. 2019; 32 (2):e00084-18. https://doi.org/10.1128/CMR.00084-18
11. AbdessemedD., Agoltsov V. A., Veselovsky S. Yu., PopovаO. M., Krasnikova E. S., Semyvolos A. M., Devrishov D. A. Importance of cellular immunity factorsin application of the environmentally safe split-conjugated anti-brucellosis vaccine in combination with immunomodulators. Theoretical and Applied Ecology. 2020; (2): 172–179. https://doi.org/10.25750/1995-4301-2020-2-172-179 (in Russ.)
12. Celli J. The intracellular life cycle of Brucella spp. Microbiology Spectrum. 2019;7:10.1128/microbiolspec.bai-0006-2019. https://doi.org/10.1128/microbiolspec.bai-0006-2019
13. Sakidibirov O. P., Dmitriev A. F. Criteria for assessing the essence of functioning infectious parasitic systems of chronic infections. Daghestan GAU Proceedings. 2023; (2): 126–130. https://elibrary.ru/nakndr (in Russ.)
14. Avila-Calderón E. D., Flores-Romo L., Sharon W., Donis-Maturano L., Becerril-García M. A., Arreola M. G. A., et al. Dendritic cells and Brucella spp. interaction: the sentinel host and the stealthy pathogen. FoliaMicrobiologica. 2020; 65 (1): 1–16. https://doi.org/10.1007/s12223-019-00691-6
15. Çelik M., Ceylan M. R., Altındağ D., Dinçer N. G., Alkan S. Diagnostic significance of hematological parameters in brucellosis. Journal of Clinical Medicine of Kazakhstan. 2023; 20 (1): 50–55. https://doi.org/10.23950/jcmk/12929
16. Moreno E., Barquero-Calvo E. The role of neutrophils in brucellosis. Microbiology and Molecular Biology Reviews. 2020; 84 (4):e00048-20. https://doi.org/10.1128/MMBR.00048-20
17. Kulakov Yu. K. Molecular mechanisms of Brucella persistence. Journal of Microbiology, Epidemiology and Immunobiology. 2018; 95 (4): 68–76. https://doi.org/10.36233/0372-9311-2018-4-68-76 (in Russ.)
18. Gorchakova N. G. Features of the parasitic system of brucellosis. Journal of Scientific Research Publications. 2017; (4): 14–27. https://elibrary.ru/yqdcts (in Russ.)
19. Alimov A. M., Zakirova L. A. Data of cell immunity at guinea pigs through vaccination and experimental brucellousinfection. ScientificNotes Kazan Bauman State Academy of Veterinary Medicine. 2016; 227 (3): 4–7. https://elibrary.ru/wmapzf (in Russ.)
20. Degtyarenko L. V., Vlasenko V. S., Sclyarov O. D. The estimation of immunological tests at dogs’ brucellosis caused by B. canis. Veterinariya. 2016; (7): 60–63. https://elibrary.ru/wjdhhb (in Russ.)
21. Degtyarenko L. V., Vlasenko V. S., Bronnikov V. S. Immunogenesis mechanismsin Brucella-sensitized guinea pigs. Vestnik veterinarii. 2015; (2): 42–46. https://elibrary.ru/tvqmnx (in Russ.)
22. Ivanov N. P. Scientific bases for the development of Brucella-based diagnostica: Author’s abstract of thesis for degree of Dr. Sci. (Veterinary Medicine). Kazan; 1984. 41 p. (in Russ.)
23. Degtyarenko L. V., Gordienko L. N., Vlasenko V. S., Gulyukin M. I., Albertyan M. P., Iskandarov M. I., et al. Methods of immunological evaluation of animalssensitized with altered forms of Brucella: a methodological guide. Moscow; Omsk: LITERA; 2017. 30 p. https://elibrary.ru/zenyrb (in Russ.)
24. Khitrik N. M. Functional activity of phagocytes in patients with herpes simplex virus infection: Author’s abstract of thesis for degree of Cand. Sci. (Medicine). Moscow; 2007. 28 p. (in Russ.)
25. Vlasenko V. S., Borisov E. S., Kosobokov E. A. Assessment of effectiveness of immune responsesto the administration of immunobiologicals. Certificate of official registration for computer software No. 2023611548 Russian Federation. Omsk Agrarian Scientific Center. No. 2023610734. Date of filing: 23.01.2023. Date of publication: 23.01.2023. Bull. No 2. (in Russ.)
26. ManakovaO.O., Yanchenko T.A., Vlasenko V. S. Testing of experimental brucellosis antigens in a stimulated cell test with nitroblue tetrazolium. Bulletin of NSAU (Novosibirsk State Agrarian University). 2024; (1): 212–218. https://doi.org/10.31677/2072-6724-2024-70-1-212-218 (in Russ.)
27. Manakova O. O., Yanchenko T. A., Vlasenko V. S. Features of oxygen-independent metabolism of neutrophils in the animals sensitized with non-agglutinogenic Brucella strain. Siberian Herald of Agricultural Science. 2024; 54 (5): 81–88. https://doi.org/10.26898/0370-8799-2024-5-8 (in Russ.)
About the Authors
O. O. ManakovaRussian Federation
Olga O. Manakova, Junior Researcher, Laboratory of Specific Prevention of Brucellosis, Department of Veterinary Medicine
26 Korolev ave., Omsk 644012
T. A. Yanchenko
Russian Federation
Tatiana A. Yanchenko, Cand. Sci. (Biology), Leading Researcher, Laboratory of Specific Prevention of Brucellosis, Department of Veterinary Medicine
26 Korolev ave., Omsk 644012
V. S. Vlasenko
Russian Federation
Vasily S. Vlasenko, Dr. Sci. (Biology), Professor, Chief Researcher, Laboratory of Epizootology and Tuberculosis Control, Department of Veterinary Medicine
26 Korolev ave., Omsk 644012
Review
For citations:
Manakova O.O., Yanchenko T.A., Vlasenko V.S. Functional and metabolic activity of neutrophils in young cattle sensitized with a non-agglutinogenic strain of Brucella. Veterinary Science Today. 2025;14(1):62-68. https://doi.org/10.29326/2304-196X-2025-14-1-62-68



































