Scroll to:
Immunogenic activity of “ARRIAH-AviFluVac” vaccine against high-pathogenicity H5N1 avian influenza virus relevant for Russia in 2023
https://doi.org/10.29326/2304-196X-2025-14-1-47-54
Abstract
Introduction. Vaccination against high-pathogenicity avian influenza (HPAI) is a well-proven way to control the disease. Inactivated whole-virion products are the most popular among the influenza vaccines. It is important to study immunogenicity of “ARRIAH-AviFluVac” vaccine against currently circulating HPAI viruses.
Objective. To assess immunogenic activity of “ARRIAH-AviFluVac” inactivated vaccine against high-pathogenicity avian influenza virus (H5N1 subtype) which was relevant for Russia in 2023.
Materials and methods. For testing purposes 4 vaccine dilutions were prepared containing whole and diluted H5 avian influenza virus antigen (1/25, 1/50 and 1/100). Each diluted sample was used to vaccinate a separate group of 4-week-old chickens. On day 28 post vaccination, the chickens were challenged with avian influenza virus A/gull/Kirov/998-1/2023 H5N1, which was isolated during an outbreak in the Russian Federation and was phylogenetically defined as high-pathogenicity agent belonging to the Asian genetic lineage of HPAI subtype H5 (clade 2.3.4.4b). Dead and sick chickens were reported in the infected groups for 6 days.
Results. The chickens vaccinated with a whole antigen dose were found to be completely protected from the clinical signs after the challenge. A decrease in the antigen concentration in the vaccine volume decreased the vaccine-induced protection. The mortality rate after the challenge of control (intact) chickens was 10/10. An analysis of the dependence of the vaccine protectivity on the volume of the antigen immunizing dose showed that one inoculation dose contained 97 PD50. An analysis of the link between protection and strength of the post-vaccination humoral immunity allowed to calculate that the expected mean antibody titer in the group, which corresponds to 90% protection in the vaccinated birds, was 5.7 log2 , or ≈ 1:52.
Conclusion. “ARRIAH-AviFluVac” vaccine demonstrates high immunogenicity against
Keywords
For citations:
Moroz N.V., Dolgov D.L., Frolov S.V., Grekhneva A.D., Kulakov V.Yu. Immunogenic activity of “ARRIAH-AviFluVac” vaccine against high-pathogenicity H5N1 avian influenza virus relevant for Russia in 2023. Veterinary Science Today. 2025;14(1):47-54. https://doi.org/10.29326/2304-196X-2025-14-1-47-54
INTRODUCTION
High-pathogenicity avian influenza (HPAI) is now a matter of concern for poultry farming all over the world. HPAI (H5N1) virus is the cause of devastating epizooties that cause significant economic damage. For example, 11 million birds had been detroyed in France by March 2022 due to HPAI (H5N1) spread; and, by September 2022, losses in the United States had exceeded 20 million chickens [1][2]. Totally, 67 countries on five continents reported HPAI (H5N1) outbreaks in 2022, resulting in loss of more than 131 million poultry [3]. From April to June 2023, HPAI (H5N1) outbreaks were reported in 25 European countries in domestic and wild birds, with a total of 98 and 634 episodes, respectively [4].
As of 17 October 2023, the following HPAI (H5N1) outbreaks were registered in the Russian Federation, as the Rosselkhoznadzor reported: 57 settlements – in wild birds; 6 settlements – on poultry farms; 8 settlements – in backyard poultry [5]. It was noted that the disease affected atypical wild avian species that year, namely seagulls. For example, there was an outbreak at the Borisovskiye Prudy in Moscow with dead seagulls detected. H5N1 subtype virus genome was isolated from the remains found there [6]. In the central regions of Russia, HPAI-infected poultry farms are all located in the immediate vicinity of the settlements where HPAI (H5N1) outbreaks were recorded in wild birds [5], which clearly indicates the source of the virus spread.
The influenza large-scale spread is primarily explained by the pathogen characteristics. At the synthesis stage in an infected cell, viral RNA does not have a repair mechanism and retains all possible “errors” in the structure, which with a probability of at least 1/10⁶ determine changes in the virus phenotype [7]. Compared to DNA-viruses with the maximum probability of error during genome replication of 1/10⁹, this is a three times difference. Each round of RNA-virus replication generates a mixed population with many variants, most of which are not viable, but some of them contain mutations that can become dominant under appropriate conditions [8][9]. At the phenotypic level, these may be changes in antigenic properties and/or changes in the pathogen tropism. In the first case, the modified agent can evade the immune response of the macroorganism, in the second case, it can increase virulence.
We emphasize that the influenza virus genome is represented by independent RNA fragments (8 fragments). If one cell is infected with various virus variants, recombination may occur, i.e. the exchange of genome fragments, which will lead to qualitative changes in the pathogen properties, including a change of the host specificity [1]. For example, in June 2023, influenza A (H5N1) virus was detected in 24 domestic cats in Poland. Infected animals showed neurological and respiratory signs, and in some cases, death was reported. In July 2023, two human cases of influenza A virus subtype H5N1 were reported in the UK, and in two cases influenza A virus subtype H9N2 was isolated [4].
Thus, the scheme depicting known avian influenza virus ecological niches (Fig. 1) only partially reflects the natural habitat of the pathogen [10].
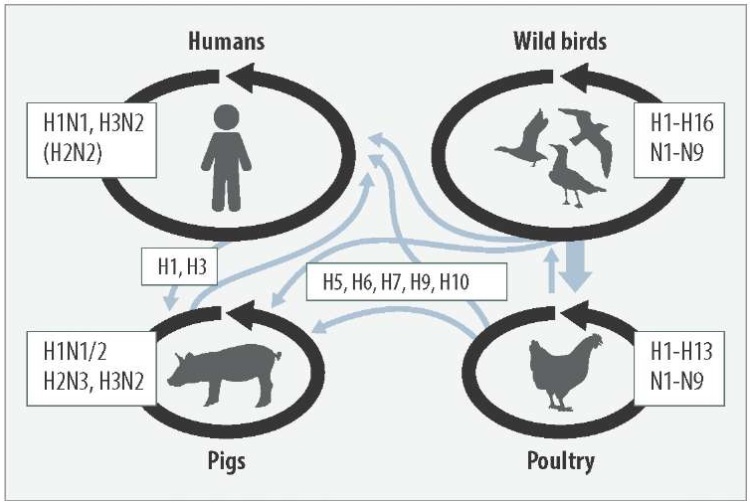
Fig. 1. Ecology of influenza A virus ([10] with changes). Hemagglutinin (H) and neuraminidase (N) variants are indicated. Black arrows show the pathogen circulation in host species, gray arrows show the virus interspecies spread
Along with restrictive measures, specific prevention is a well-proven way to control HPAI. Inactivated whole-virion vaccines are the most popular among anti-influenza vaccines [11][12]. Protectivity of such vaccines depends on two related factors: the antigen concentration in the vaccine and the structural correspondence between the vaccine antigens and the field isolate [12][13]. At the same time, for biosafety reasons it is recommended to use low-pathogenicity virus variants to obtain antigens [14]. “ARRIAH-AviFluVac” is an example of an inactivated vaccine for specific prevention of HPAI based on a low-pathogenicity virus variant.
The objective of the research was to assess effectiveness of “ARRIAH-AviFluVac”, the inactivated vaccine against HPAI H5N1 which caused local outbreaks in several regions of Russia in 2023.
To achieve the objective the following tasks were set:
– to determine phylogenetic type of the HPAI isolate recovered in the outbreak in the Russian Federation, which will be used to test the vaccine protective effect;
– to assess 50% protective dose contained in the inoculation volume;
– to calculate post-vaccination antibody titer that protects 90% of the vaccinated birds.
MATERIALS AND METHODS
The research object: “ARRIAH-AviFluVac” inactivated emulsion vaccine against avian influenza (H5). The antigen concentrations (D) in the vaccine inoculation dose (represented by “Yamal” production strain of low pathogenicity avian influenza H5 virus) were regulated by diluting it with saline solution in the ratios of 1/25, 1/50 and 1/100. When preparing vaccine samples, the active component (antigen) was combined with an oil adjuvant in a ratio of 30:70 (by weight) and emulsified on a high-speed Silverson laboratory mixer (Great Britain) at a speed of 6,000 rpm for 5 minutes. The emulsion stability after mixing was evaluated after centrifugation at 1,000 g for 10 min. The emulsion was considered stable, if creaming of the light (oil) fraction did not exceed 5% by volume, and no creaming of the heavy (water) fraction was observed.
Thus, vaccine samples were prepared containing a whole antigen (D = 1) and antigens diluted at 1/25, 1/50 and 1/100 (D = 25, D = 50 and D = 100) from the initial concentration.
Poultry. For the experiment, avian influenza virus seronegative 4-week-old Lohmann Brown cross-breds were used. The chickens were handled in accordance with GOST 33215-2014 and in accordance with Directive 2010/63/EU (dated 22.09.2010) on protection of animals used for scientific purposes.
Vaccination. Each vaccine sample was tested in a separate group of 10 chickens. The vaccine was injected intramuscularly into the chest area at a dose of 0.5 cm³. Additionally, a virus control group was formed (10 chickens), where no vaccination was performed (intact chickens). Chicken groups were kept in isolated rooms with autonomous ventilation, water and feed supply.
Chicken embryos. SPF chicken embryos (9–11-day-old) were used for the research (VALO BioMedia GmbH, Germany).
Isolation of avian influenza virus. The pathological material obtained from the seagulls that died of HPAI was used. A 10% tissue suspension was prepared on a phosphate buffer (pH 7.2–7.4), which was centrifuged for 15 min at 1000 g. Antibiotics were added to the supernatant (100 U/mL of benzylpenicillin sodium salt, 100 µg/mL of streptomycin sulfate and 50 U/mL of nystatin). The resulting material was injected into the allantoic cavity of chicken embryos in a volume of 0.2 cm³. Embryos were incubated at a temperature of 37 °C and a relative humidity of 60–70% with ovoscopy done daily. The embryos that died 24 hours after incubation or more were used for harvesting extraembryonic fluid. The death specificity was confirmed by hemagglutinating activity in the hemagglutination test and by identification of hemagglutination inhibition with a specific serum [15].
Calculating virus titer in chicken embryos. Method of limiting dilutions was used. Serial tenfold dilutions of the virus material in a phosphate buffer (pH 7.2–7.4) were prepared. Each dilution was tested in a group of embryos (n ≥ 5). The material was inoculated into the allantoic cavity in a volume of 0.2 cm³. The virus presence, which means i.e. a positive reaction, was confirmed, if the embryo death was observed after more than 24 hours of incubation. The titer was calculated according to Karber and expressed as EID50/cm³.
Reverse transcription polymerase chain reaction (RT-PCR). The total RNA was isolated using RNeasy Mini Kit (QIAGEN, the Netherlands, cat. No. 74106) in accordance with the manufacturer’s instructions. One RT-PCR stage was performed using OneStep RT-PCR Kit (QIAGEN, the Netherlands, cat. No. 210212) with appropriate primer systems for detecting avian influenza virus genome and identifying H5N1 subtype.
Sequencing the virus genome. Nucleotide sequences of the gene fragments were determined using ABI Prism 3130 automatic sequencer (Applied Biosystems, USA). BioEdit application software package, Version 7.0.5.3, was used to analyse and compare nucleotide and corresponding amino acid sequences. Sequences of isolates and strains of A/H5 avian influenza virus previously published in the international GenBank database were also used for comparative analysis (https://www.ncbi.nlm.nih.gov/genomes/FLU/Database). The phylogenetic tree was constructed and edited using NJ algorithm in MEGA package, Version 7.
Hemagglutination assay (HA assay). Samples of antigen-containing materials were examined in HA assay according to the procedure described in instructions for a hemagglutination inhibition test kit used to detection of antibodies to avian influenza virus subtype H5. The titer of hemagglutinating units was calculated.
Hemagglutination inhibition test (HI test). Avian blood serum samples were tested in HI test in accordance with the instructions for the hemagglutination inhibition test kit used for detection of antibodies to avian influenza virus subtype H5 (Federal Centre for Animal Health, Russia) [16]. The antibody titer was calculated. The result was considered positive, if the titer was 1:16 and more, that is 4 log2.
Challenge. Immunized and intact birds were challenged on day 28 post vaccination. HPAI strain A/gull/Kirov/998-1/2023 H5N1 was used for the challenge at a dose of 6.0 lg EID50. The viral material was injected intramuscularly into the thigh area in a volume of 0.5 cm³. Clinical status of the challenged birds was monitored for 10 days.
Processing experimental data. Conventional methods were used to process the set of variables (mean values, standard deviations, and standard errors of the mean were calculated). Elements of regression analysis were used. Special statistical methods are described in the text. Calculations were done and the graphs were drawn using Excel application.
RESULTS AND DISCUSSION
Virus isolation, virulence assessment and phylogenetic analysis. It was found that the tested biological material contained the infectious virus that was lethal to the embryos (specific mortality was 23/30). Samples of extraembryonic fluid tested positive in HA assay (from 1:64 to 1:256) and RT-PCR revealed high concentraiton of avian influenza virus genome in them (mean Ct value = 18).
The hemagglutinin cleavage site of the isolated avian influenza virus had a structure -REKRRKR-, which made it possible to characterize is as potentially highly virulent.
Virus-containing extraembryonic fluid intravenously injected to ten 5-week-old chickens, seronegative to the avian influenza virus, resulted in death of 9 chickens (90%) during the following 10 days after injection (i.e. 10-fold dilution in phosphate buffer was used and each chicken received 0.1 cm³). The dead chickens showed typical clinical signs of HPAI (diarrhea, nasal discharge, cyanosis of unfeathered skin areas). The death specificity was confirmed by RT-PCR, which revealed HPAI virus genome in the biological material. The results obtained corresponded to the clinical signs of HPAI [14].
Comparative genetic analysis of hemagglutinin fragment nucleotide sequences revealed that the virus belongs to the Asian genetic lineage of HPAI virus subtype H5 (clade 2.3.4.4.b), which earlier became epizootic for Asia, Europe, Africa, North and South America. The isolated virus was identified as avian influenza virus strain A/gull/Kirov/998-1/2023 H5N1. The strain position in the phylogenetic tree is shown in Figure 2.
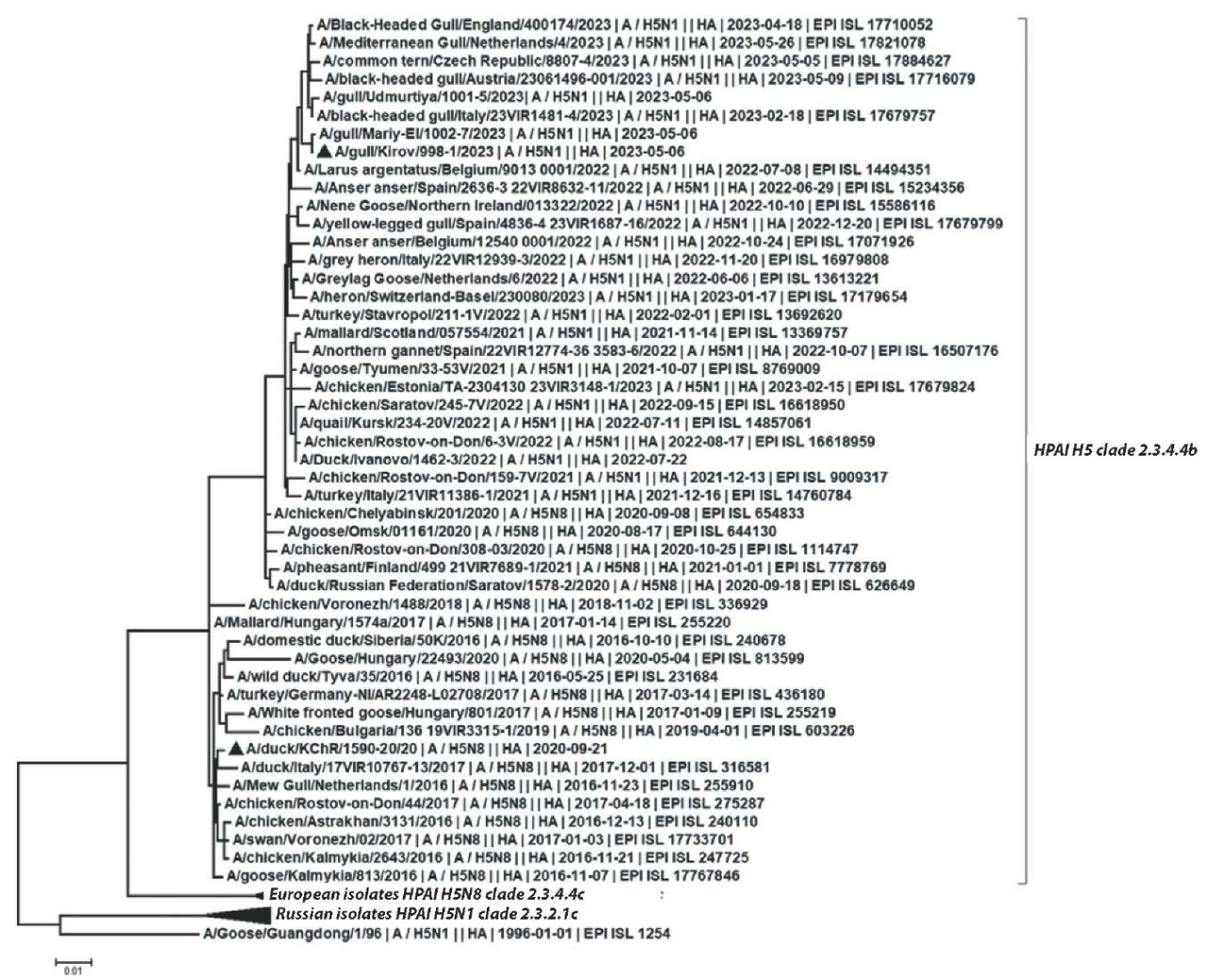
Fig. 2. A phylogenetic tree based on the full-length hemagglutinin gene sequences
According to GenBank and GISAID (EpiFlu) databases, the most genetically related to A/gull/Kirov/998-1/2023 H5N1 are H5N1 subtype viruses detected in 2023 in several European countries. Taking into account the time of detection in the European countries (i.e. February – May 2023) and comparing the data to the GISAID database (EpiFlu), identical isolates had been actively circulating for several months, at least, since the beginning of 2023.
Thus, taking into account spread of H5N1 influenza virus in the region, as well as introduction and spread of infection in a number of RF regions, A/gull/Kirov/998-1/2023 H5N1 virus strain was used in further work.
Assessing vaccine immunogenicity. All vaccine samples containing certain antigen concentrations were tested in birds in parallel. On day 28 post vaccination, mean log2 antibody titer against avian influenza virus was calculated in HI test (log2 T).
Further, all experimental groups were infected with the A/gull/Kirov/998-1/2023 H5N1 strain. For 10 days, current clinical indicators were examined daily in each group (c = a + b, where a and b are the number of clinically diseased and dead birds, respectively). At the end of the observation, the accumulated clinical indicators in the groups were assessed (∑c/n, where n is the number of birds in the group before infection) and the protective activity of the vaccine type P = (1 – ∑c/n) × 100 was calculated.
Indicators of the vaccine immunogenicity established in experimental groups are shown in the Table.
Table
Indicators of vaccine immunogenicity against H5N1 avian influenza virus
|
Indicators according to the tested antigen concentrations |
|||
|
Antigen concentration, D* |
HI titre |
Clinical indicator |
Protective activity (P), % |
|
log2 T** |
∑c/n*** |
P = (1 – ∑c/n) × 100 |
|
|
1 |
6.67 |
0/10 |
100 |
|
1:25 |
5.33 |
1/10 |
90 |
|
1:50 |
4.33 |
4/10 |
70 |
|
1:100 |
2.00 |
6/10 |
50 |
|
control |
0.47 |
10/10 |
0 |
|
* antigen concentration in the inoculation volume; ** mean log antibody titer in the group of the vaccinated chickens, n = 3; *** ∑c – number of clinically diseased and dead birds after the challenge [indicator assessed during the experiment]; n – number of chickens in the group before the challenge. |
|||
The relationship between the antigen concentration in the inoculation volume (D) and the vaccine protective activity (P) was studied. Regression analysis was used to construct the most probable model of the relationship between the antigen concentration and the vaccine protective activity [17]. The following linear regression equation was drawn up: P = (–0.5184) D + 100.31 (R² = 0.98), where P is the predicted index value corresponding to the given D.
Correlation regression analysis was used to graphically represent the regression of P and D. The results are shown in Figure 3.
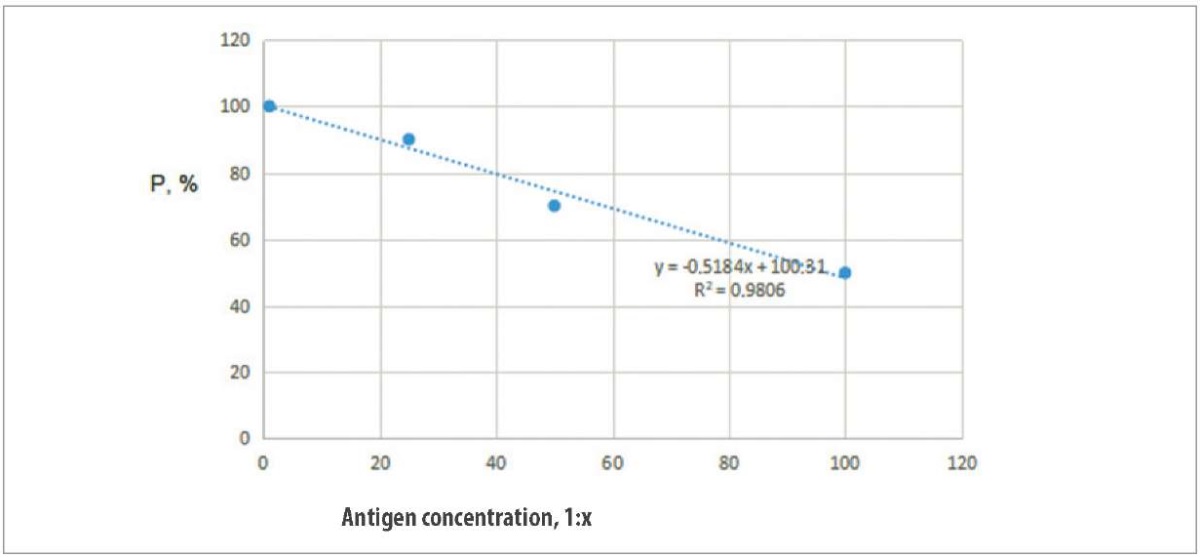
Fig. 3. Relationship between the tested antigen concentrations and protectivity of the vaccine against HPAI H5N1
Using the regression equation, it was calculated that the antigen concentration providing protection for 50% (PD50) of the vaccinated poultry is 97, which corresponds to initial antigen concentration of 1:97. Thus, the vaccine protectivity is 97 PD50, which is consistent with the requirements of the World Organization for Animal Health, i.e. PD50 ≥ 50.
Studying relationship between vaccine protectivity and humoral antibody titers. The relationship between humoral antibody titers (T, log2) and vaccine protectivity was analysed (P, %), using the data obtained for the tested antigen concentrations. The results are shown in Figure 4.
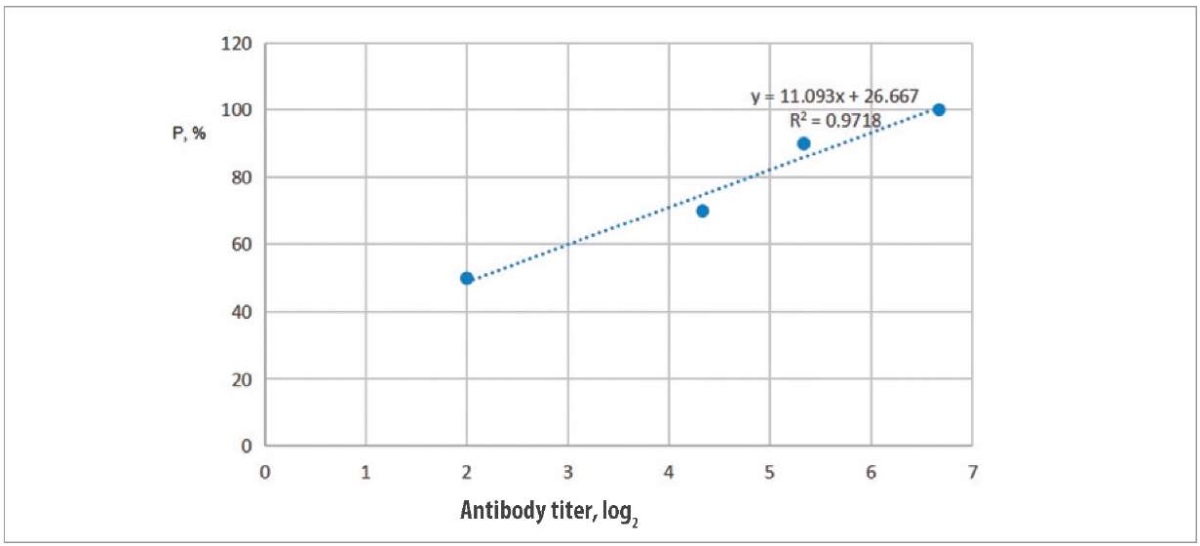
Fig. 4. Relationship between H5 virus antibody titer and level of the vaccine protectivity
Regression line P by log2 T is shown, where ‘P is the index predicted by equation ‘P = (11.093) log2 T + 26.667, with an adequacy score of R² = 0.97.
The resulting equation allowed us to calculate the expected antibody titer (T90), which corresponds to 90% protection of the vaccinated poultry. The desired estimate was log2 T90 = 5.7.
It is known that HPAI outbreaks on poultry farms (for example, in the USA) mostly coincide geographically with the migration routes of wild waterfowl [1]. Given the role of avifauna in the disease spread in some regions of the Russian Federation, A/gull/Kirov/998-1/2023 H5N1 strain should be considered epizootically dangerous. Therefore, it is justified to use the strain for challenge purposes to assess the protectivity of the vaccine against H5N1.
The antigen concentration in the inoculation volume of the inactivated vaccine is the most important characteristic of the vaccine. The major antigen (hemagglutinin) can be measured in absolute units, such as weight units [11], or in units of action, such as PD50. The inoculation volume of an effective avian influenza vaccine is considered to contain 50 PD50 [12][14], which corresponds to 0.3–7.8 [12] or 3 µg of hemagglutinin [14].
The challenge procedure fully corresponded to the generally accepted method [14]. The results obtained demonstrate that the inoculation volume of “ARRIAH-AviFluVac” contained 97 PD50, which ensured protection of 100% of the immunized poultry who received one inoculation volume, thus, proving the possibility to practically use it in accordance with the attached instructions. This means that the antigenic potential of “ARRIAH-AviFluVac” vaccine against A/gull/Kirov/998-1/2023 H5N1 strain is 1.9 times higher than the recommended protective activity [14].
Previously, protective properties of a “Yamal” strain-based vaccine [18] were analysed and the vaccine induced effective protection against heterologous HPAI H5N8 subtype (A/duck/KChR/1590-20/2 strain). A/duck/KChR/1590-20/2 virus also belongs to genetic clade 2.3.4.4b. The results of the phylogenetic analysis given in this work indicate an active antigenic drift of influenza A virus subtype H5N1 in Eurasia in 2016–2023 (Fig. 1). Those avian influenza virus isolates against which the “Yamal” strain-based vaccine has proved to be effective, are shown in Figure 2 in a black triangle.
Since poultry are protected from generalized avian influenza mainly by antibodies developed against viral hemagglutinin [19], HI test data are important to indicate post-vaccination humoral antibody level. HI antibody titer of 4 log2 is known to protect the poultry from infection and death [14][19], and antibody titer of 6.5 log2 prevents, inter alia, local replication of the virus [20]. At the same time, it is required that the antibody titer should be more than 4 log2 in 80% of the poultry population [21]. Within this research, 90% protection of the vaccinated birds corresponded to the expected titers of 5.7 log2 for “ARRIAH-AviFluVac” vaccine.
CONCLUSIONS
The following conclusions can be made based on the obtained results.
- The virus isolated from the pathological material was identified as H5N1 A/gull/Kirov/998-1/2023 strain, which belongs to the Asian genetic lineage of H5 subtype (clade 2.3.4.4b). The strain is characterized as epizootically dangerous for the Russian Federation.
- It has been established that “ARRIAH-AviFluVac” inactivated vaccine ensures protection from HPAI virus strain A/gull/Kirov/998-1/2023 (H5N1) and, on day 28 after vaccination, it completely prevents clinical manifestation of the disease during challenge. The protective potential of the vaccine was 97 PD50 in one inoculation dose.
- It is shown that “ARRIAH-AviFluVac” vaccine induces intense humoral immunity against influenza in poultry on day 28 after administration. The post-vaccination HI antibody titer to HPAI, corresponding to protection of 90% of the vaccinated poultry, was as predicted i.e. 5.7 log2.
References
1. Mailyan E. S. Gripp ptits. Novyi vzglyad na proshloe, nastoyashchee i budushchee ptitsevodstva = Avian Influenza. A new look at the past, present and future of poultry farming. NPK Farmindustria. https://pharmindustria.com/projects/poleznye-stati-po-veterinarii/gripp-ptits-novyyvzglyad-na-proshloe-nastoyashchee-ptizevodstva (in Russ.)
2. U. S. Centers for Disease Control and Prevention (CDC). Current Situation: Bird Flu in Poultry. https://www.cdc.gov/bird-flu/situation-summary/current-bird-flu-situation-in-poultry.html?CDC_AAref_Val=https://www.cdc.gov/flu/avianflu/poultry.htm
3. FAO. Ongoing avian influenza outbreaks in animals pose risk to humans: Situation analysis and advice to countries from FAO, WHO, WOAH. 12.07.2023. https://www.fao.org/animal-health/news-events/news/detail/ongoing-avian-influenza-outbreaks-in-animals-pose-risk-to-humans/en
4. European Food Safety Authority, European Centre for Disease Prevention and Control, European Union Reference Laboratory for Avian Influenza, Adlhoch C., Fusaro A., Gonzales J. L., et al. Avian influenza overview April – June 2023. EFSA Journal. 2023; 21 (7):e08191. https://doi.org/10.2903/j.efsa.2023.8191
5. Avian influenza outbreaksin the Russian Federation in 2023 (according to the WHO data as of 17.10.2023). https://fsvps.gov.ru/wp-content/uploads/2023/10/%D0%92%D0%9F%D0%93%D0%9F-%D0%BD%D0%B0-17.10.2023-scaled.jpg (date of access: 03.07.2024). (in Russ.)
6. Comment from the Rosselkhoznadzor Administration for the city of Moscow, Moscow and Tula Oblasts on identification of HPAI cases in the city of Moscow. 18.05.2023. https://777.fsvps.gov.ru/news/kommentarij-upravlenija-rosselhoznadzora-po-g-moskva-moskovskoj-i-tulskoj-oblastjam-po-vyjavleniju-sluchaev-vysokopatogennogo-grippa-ptic-na-territorii-g-moskvy/?ysclid=m3y8yxbxy957274774 (in Russ.)
7. Suárez P., Valcárcel J., Ortín J. Heterogeneity of the mutation rates of influenza A viruses: isolation of mutator mutants. Journal of Virology. 1992; 66 (4): 2491–2494. https://doi.org/10.1128/jvi.66.4.2491-2494.1992
8. Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982; 215 (4540): 1577–1585. https://doi.org/10.1126/science.7041255
9. Webster R. G., Bean W. J., Gorman O. T., Chambers T. M., Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiological Reviews. 1992; 56 (1): 152–179. https://doi.org/10.1128/mr.56.1.152-179.1992
10. Long J. S., Mistry B., Haslam S. M., Barclay W. S. Host and viral determinants of influenza A virusspeciesspecificity. Nature ReviewsMicrobiology. 2019; 17 (2): 67–81. https://doi.org/10.1038/s41579-018-0115-z
11. Peyre M., Fusheng G., Desvaux S., Roger F. Avian influenza vaccines: a practical review in relation to their application in the field with a focus on the Asian experience. Epidemiology and Infection. 2009; 137 (1): 1–21. https://doi.org/10.1017/s0950268808001039
12. Swayne D. E. Laboratory methods for assessing and licensing influenza vaccines for poultry. In: Animal Influenza Virus. Methods and Protocols. Ed. by E. Spackman. 3rd ed. New York: Humana Press; 2020; Chapter 16: 211–225. https://doi.org/10.1007/978-1-0716-0346-8_16
13. Lee Y. J., Sung H. W., Choi J. G., Lee E. K., Jeong O. M., Kwon Y. K, et al. Effects of homologous and heterologous neuraminidase vaccines in chickens against H5N1 highly pathogenic avian influenza. Avian Diseases. 2007; 51 (Suppl. 1): 476–478. https://doi.org/10.1637/7548-033106R.1
14. Avian influenza (including infection with high pathogenicity avian influenza viruses). In: WOAH. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2021; Сhapter 3.3.4. https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.03.04_AI.pdf
15. Chvala I. A., Manin T. B., Mudrak N. S., Drygin V. V. Guidelines for isolation of avian influenza virusin chicken embryos: approved by the Federal Centre for Animal Health No. 59-08. Vladimir; 2008. 9 p. (in Russ.)
16. Instructions for the use of a HI kit for detection of antibodies to avian influenza virussubtypeH5. https://shop.arriah.ru/upload/iblock/ad5/rwd6v5w4evkwfcv1l1ba1kck0zb13zkp.pdf (in Russ.)
17. Urbakh V. Yu. Statistical analysis in biological and medical research. Moscow: Medicina; 1975. 297 p. (in Russ.)
18. Frolov S. V., Chvala I. A., MorozN. V., KulakovV. Yu., SosipatorovaV. Yu., Andreychuk D. B. Immunobiological properties of inactivated anti-highly pathogenic avian influenza vaccines based on antigens of А/Н5N1 avian influenza virusstrains of different virulence. Veterinary Science Today. 2022; 11 (4): 367–374. https://doi.org/10.29326/2304-196X-2022-11-4-367-374
19. Katz J. M., Lu X., Frace A. M., Morken T., Zaki S. R., Tumpey T. M. Pathogenesis of and immunity to avian influenza A H5 viruses. Biomedicine & Pharmacotherapy. 2000; 54 (4): 178–187. https://doi.org/10.1016/s0753-3322(00)89024-1
20. Kumar M., Chu H.-J., Rodenberg J., Krauss S., Webster R. G. Association of serologic and protective responses of avian influenza vaccines in chickens. Avian Diseases. 2007; 51 (Suppl. 1): 481–483. https://doi.org/10.1637/7605-041706R1.1
21. Javadov E. J., Dmitrieva M. E. Avian influenza. Saint Petersburg; Lomonosov: All-Russian ResearchVeterinary Institute of Poultry Science; 2011. 188 p. https://elibrary.ru/ccythi (in Russ.)
About the Authors
N. V. MorozRussian Federation
Natalia V. Moroz, Cand. Sci. (Veterinary Medicine), Head of Laboratory for Avian Diseases Prevention
Yur’evets, Vladimir 600901
D. L. Dolgov
Russian Federation
Dmitry L. Dolgov, Cand. Sci. (Veterinary Medicine), Head of Sector, Laboratory for Avian Diseases Prevention
Yur’evets, Vladimir 600901
S. V. Frolov
Russian Federation
Sergey V. Frolov, Cand. Sci. (Veterinary Medicine), Head of Department for Avian Disease Prevention
Yur’evets, Vladimir 600901
A. D. Grekhneva
Russian Federation
Alena D. Grekhneva, Postgraduate Student, Leading Specialist, Head of Reference Laboratory for Avian Viral Diseases
Yur’evets, Vladimir 600901
V. Yu. Kulakov
Russian Federation
Vladimir Yu. Kulakov, Cand. Sci. (Veterinary Medicine), Leading Researcher, Laboratory for Avian Diseases Prevention
Yur’evets, Vladimir 600901
Review
For citations:
Moroz N.V., Dolgov D.L., Frolov S.V., Grekhneva A.D., Kulakov V.Yu. Immunogenic activity of “ARRIAH-AviFluVac” vaccine against high-pathogenicity H5N1 avian influenza virus relevant for Russia in 2023. Veterinary Science Today. 2025;14(1):47-54. https://doi.org/10.29326/2304-196X-2025-14-1-47-54



































