Scroll to:
Role of rotavirus, coronavirus and Escherichia coli in disease etiology in young cattle (review)
https://doi.org/10.29326/2304-196X-2025-14-1-14-23
Abstract
Introduction. One of the most prevalent groups of pathologies detected in young cattle involves gastrointestinal diseases. They are often caused by infectious agents, among which rotavirus, coronavirus and pathogenic Escherichia coli are predominant.
Objective. Analysis and systematization of up-to-date information on the role of rotavirus, coronavirus and pathogenic Escherichia coli strains in the etiology of diseases of cattle, including young animals, data on the incidence of these infections in the Russian Federation and other countries of the world as well as relevance of vaccination against the above-mentioned pathogens.
Results. The paper provides information on the structure of rotavirus, coronavirus and Escherichia coli, on the biological properties of the pathogens, and factors affecting the disease form and severity. Based on the analysis of domestic and foreign scientific publications, data on the prevalence of colibacillosis, rotavirus and coronavirus infections are presented, and the main methods of their control are described. The significance of the vaccines for the prevention of these diseases is confirmed, the factors influencing the vaccine prevention effectiveness are listed, and measures to increase it are given.
Conclusion. The average global incidence of rotavirus infection is 32.7%, coronavirus infection is 18.4%, and colibacillosis is 39.1%. In Russia, the prevalence rate of the above-mentioned diseasesis 41.4, 33.1 and 30.2%, respectively. Thus, in the Russian Federation, the incidence of bovine rotavirus and coronavirus infections exceeds the global average by 8.7 and 14.7%, respectively. The colibacillosis situation in Russia is better than in most countries: the disease is reported by 8.9% less frequently than the globala verage. High genetic diversity and prevalence of the above-mentioned pathogens require an integrated approach to their control. One of the most effective methods is vaccination, which makes the development of effective and safe vaccines against rotavirus, coronavirus and Escherichia coli infections an urgent task.
Keywords
For citations:
Kruglov I.A., Kononov A.V., Nesterov A.A., Kononova S.V., Pruntova O.V. Role of rotavirus, coronavirus and Escherichia coli in disease etiology in young cattle (review). Veterinary Science Today. 2025;14(1):14-23. https://doi.org/10.29326/2304-196X-2025-14-1-14-23
INTRODUCTION
Gastrointestinal (GI) diseases are one of the most prevalent groups of pathologies in young cattle. The GI disorders are most often associated with the lack of a complex of necessary measures to prevent infectious diseases in young animals: ineffective or untimely vaccination, untimely feeding with colostrum, non-compliance with hygienic standards in the maintenance of production facilities, faulty diet formulation and non-compliances with the feeding technics. The combination of these factors creates conditions for the development of enteritis of infectious etiology in cattle [1][2].
One of the most common causes of enteritis in young cattle is the effect of rotaviruses, coronaviruses and Escherichia coli. Therefore, they make the most significant impact on the health of calves as compared to other infectious agents that cause GI disorders, thus requiring appropriate preventive measures [3][4].
The novelty of the analytical study lies in the generalization of scientific information on the bovine disease situation caused by rotaviruses, coronaviruses and pathogenic E. coli strains in the Russian Federation and other countries of the world.
The purpose of this review is to analyze and systematize up-to-date information on the role of rotavirus, coronavirus and pathogenic E. coli strains in the etiology of bovine diseases, inter alia in young animals, data on the incidence of these infections in the Russian Federation and other countries of the world, as well as relevance of vaccine prevention of the above-mentioned pathogens.
ROLE OF ROTAVIRUSES IN THE DEVELOPMENT OF BOVINE PATHOLOGIES
The taxonomic group Reoviridae includes non-enveloped viruses containing double-stranded, segmented RNA represented by 11 segments. Rotaviruses belong to the family Sedoreoviridae. The rotavirus capsid consists of 3 layers. The outer layer is represented by VP4 and VP7 proteins, the middle one – by VP6, the inner one – by VP1, VP2 and VP3, and the virion size is 70 nm. The segmented nature of the genome is the reason for the rotavirus reassortment [2].
Since the rotaviruses are widespread, young cattle can be infected with them from their first days of life. The rotavirus infection in calves is clinically manifested by depression, diarrhea, and dehydration (Fig. 1). During the milk feeding, the feces of the diseased calves are of yellow or white color and of varied consistency (from watery to thick), however, the presence of blood in the feces is not typical for rotavirus infection. In case of secondary bacterial infection, the mortality rate of newborn calves can reach 60%. During autopsy, catarrhal or catarrhal-hemorrhagic enteritis is reported in dead animals [2].
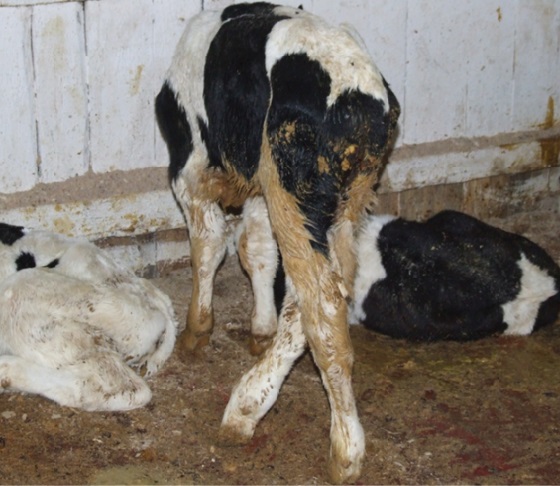
Fig. 1. Clinical manifestation of rotavirus infection, characterized by diarrhea and depression (photo from the personal archive of A. V. Kononov)
Rotavirus infection is most acute during the cold season, and the severity of the disease depends upon the decrease in the indoor temperature. The risk of severe disease is also increased by feeding with colostrum from cows that lack rotavirus antibodies as well as by the presence of other enteropathogenic infectious agents [2].
In addition to young animals, the adult animals can also be infected with rotavirus, but their infection is asymptomatic. The number of asymptomatic carriers on the infected farms can reach 44%. The infected adult animals play a significant role in the virus spread: within a few weeks, one such animal can shed up to 10¹⁰ viral particles per 1 g of feces. Since this virus has a high resistance to environmental factors, the pathogen can circulate on the farm for a long time and infect a large number of susceptible animals, including calves [2].
Enteritides of rotavirus etiology are reported in calves more often than other infectious GI diseases. On the infected farms, they can infect up to 100% of calves, and vaccination of cattle may be ineffective due to the rotavirus reassortment and emergence of new recombinant virus variants [5]. The incidence of the rotavirus infection in cattle in the world and in the Russian Federation can reach 70% or higher (Table 1 and 2).
Table 1
Bovine rotavirus infection prevalence in the countries of the world
|
Country |
Region |
Estimated prevalence, % |
Source |
|
Australia (2011) |
Australia |
79.90 |
[6] |
|
Australia (2004–2005) |
26.00 |
[7] |
|
|
China (1984–2021) |
Asia |
35.70 |
[8] |
|
Iran (2000) |
34.00 |
[9] |
|
|
Iran (1981) |
31.74 |
[10] |
|
|
Iran (2001) |
28.80 |
[10] |
|
|
Iran (2010) |
27.90 |
[10] |
|
|
Norway (2004–2007) |
Europe |
67.70 |
[11] |
|
Switzerland (2005–2006) |
58.70 |
[12] |
|
|
Spain (2000) |
43.50 |
[10] |
|
|
Spain (1998) |
42.70 |
[10] |
|
|
Turkey (2007) |
41.17 |
[10] |
|
|
Belarus (2020–2021) |
39.60 |
[13] |
|
|
Ukraine (2012) |
28.60 |
[14] |
|
|
Sweden (2003) |
13.00 |
[15] |
|
|
Sweden (1987–1988) |
5.40 |
[16] |
|
|
Argentina (1994–2003) |
South and North America |
42.00 |
[17] |
|
Brazil (2007) |
33.00 |
[10] |
|
|
USA (2010) |
12.20 |
[18] |
|
|
Brazil (2007) |
11.00 |
[19][20] |
|
|
Costa Rica (1981) |
10.00 |
[10] |
|
|
Costa Rica (1998) |
7.00 |
[10] |
Table 2
Bovine rotavirus prevalence in the Russian Federation
|
Region of the Russian Federation |
Federal district |
Estimated prevalence, % |
Source |
|
Republic of Dagestan (2001–2005) |
North Caucasian |
77.90 |
[21] |
|
Irkutsk Oblast (2020) |
Siberian |
44.40 |
[22] |
|
Irkutsk Oblast (2004–2017) |
17.60 |
[5] |
|
|
Central Black Earth Region (2017–2018) |
Central |
22.30 |
[23] |
|
12 Oblasts (2007–2011) |
Central, Volga and Far Eastern |
44.55 |
[24] |
Based on the systematized data, it can be concluded that the average prevalence of rotavirus infection in cattle in the countries of the world was 32.7% in 1981–2021.
As for the Russian Federation, the issue of the rotavirus infection prevalence in cattle also remains relevant. The data in Table 2 show that over the past 20 years, the incidence on farms in the Russian Federation has averaged 41.4%, which exceeds the same indicator in other countries by 8.7%. Such a high prevalence may be due to the non-compliances with calf housing conditions and lack of appropriate preventive measures targeted to all age groups of cattle on the farms of the Russian Federation.
ROLE OF CORONAVIRUSES IN THE DEVELOPMENT OF BOVINE PATHOLOGIES
Bovine coronavirus belongs to the Coronaviridae family, genus Betacoronavirus, and species Betacoronavirus gravedinis. The genome is represented by a single-stranded (+) RNA and it is the longest among the RNA viruses. The virion is 65–210 nm in diameter and contains a supercapsid.
Coronavirus infection is ubiquitous. During their lifetime, up to 90% of animals become infected with coronavirus. Based on the clinical picture, there are three disease forms: intestinal, respiratory, and so-called winter dysentery. The development of one disease form or another depends not on the serotype of the pathogen, but on the age of the recipient [2].
The intestinal form is most typical for young animals from the first days of life to the age of five months. It is characterized by inflammatory lesions of the large and small intestines, which lead to severe diarrhea (often with blood), as well as to high mortality rate, which can reach 20% [2][5].
The respiratory infection is typical for calves from two to six months of age, which is characterized by rhinitis, cough, fever, loss of appetite, and often by concurrent diarrhea (Fig. 2). In severe cases, dyspnea, bronchopneumonia, and weight loss up to exhaustion and death are reported [2].

Fig. 2. Clinical manifestation of сoronavirus infection, characterized by depression (photo from the personal archive of A. V. Kononov)
In adult animals, the disease occurs in the form of winter dysentery, which is characterized by the following clinical signs: profuse diarrhea (up to 100% of cases), often with blood, cough, seromucous nasal discharge, harsh rapid breathing. The disease leads to the decrease in milk yield from 25 to 90%, while restoring the previous milk yield can take from 2.5 to 4 months [25].
The adult animals can be asymptomatic coronavirus carriers, while being a source of infection transmission with feces (96%) and nasal mucus (84%). Over 70% of adult animals can shed the virus despite the presence of antibodies. This is due to the fact that a long period of persistence and shedding by the recovered animals are typical for the coronavirus [2].
Low temperatures and lesser exposure to UV rays in winter not only contribute to the pathogen persistence, but also reduce the overall level of animal resistance, which leads to 50–60% increase in the amount of virus shed into the environment, which, in turn, results in the increase in the number of cases of coronavirus infection. In addition to the time of year, the bovine infection with the coronavirus is also affected by the physiological condition of the animal, for example, during the calving period and the first two weeks after it, the number of the virions shed by the infected animal increases [2].
The virus can enter the body of calves not only by alimentary route (through contaminated surfaces or contaminated udder and perineum of cows), but also by airborne route, thus resulting in a high risk of infection [2][26].
The analysis of publications from 1995 to 2022 showed that the prevalence of bovine coronavirus in the world was 18.4% (Table 3), in Russia – 33.1% (Table 4), which is 14.7% more than the global average.
Table 3
Prevalence of bovine coronavirus infection in the countries of the world
|
Country |
Region |
Estimated prevalence, % |
Source |
|
Australia (2011) |
Australia |
21.60 |
[6] |
|
Japan (1995–1997) |
Asia |
57.00 |
[27] |
|
Iran (2010) |
3.10 |
[10] |
|
|
Norway (2004–2007) |
Europe |
39.30 |
[11] |
|
Belarus (2020–2021) |
28.70 |
[13] |
|
|
Ukraine (2012–2019) |
22.40 |
[14] |
|
|
Switzerland (2005–2006) |
7.80 |
[12] |
|
|
Spain (1998) |
7.30 |
[10] |
|
|
Turkey (2007) |
1.96 |
[10] |
|
|
Sweden (2003) |
1.00 |
[15] |
|
|
Brazil (2007) |
South and North America |
22.00 |
[10] |
|
Brazil (2007) |
16.00 |
[20] |
|
|
Costa Rica (1998) |
9.00 |
[10] |
|
|
USA (2010–2011) |
20.90 |
[18] |
Table 4
Prevalence of bovine coronavirus infection in the Russian Federation
|
Region of the Russian Federation |
Federal district |
Estimated prevalence, % |
Source |
|
Republic of Dagestan (2001–2005) |
North Caucasian |
62.60 |
[21] |
|
Siberia (2010) |
Siberian |
71.30 |
[28] |
|
Siberia (2022) |
11.80 |
[29] |
|
|
Irkutsk Oblast (2020) |
11.10 |
[22] |
|
|
Irkutsk Oblast (2004–2017) |
2.20 |
[5] |
|
|
Central Black Earth Region (2017–2018) |
Central |
26.70 |
[23] |
|
14 oblasts (2007–2011) |
Central, Volga, Southern and Far Eastern |
45.90 |
[24] |
DEPENDENCE OF THE DISEASE FORM IN YOUNG CATTLE ON E. COLI PATHOGENIC PROPERTIES
Among the bacterial diseases of young cattle, colibacillosis (escherichiosis) caused by various E. coli serovariants is the most prevalent.
E. coli bacteria have a complex antigenic structure comprising three types of antigens: somatic O-antigen (contains 164 variants); capsular K-antigen (90 variants) and flagellar H-antigen (55 variants). These antigens in various combinations make more than 9,000 serovariants, 170 of which demonstrate pathogenic properties [2][30].
E. coli strains that cause animal diseases have various pathogenicity factors, which include polysaccharides, adhesins, enterotoxins, etc. Their functions include the following: hindering and weakening the immune response (capsular polysaccharides), destruction of body cells (enterotoxins), attachment of bacteria to the surface of susceptible cells (adhesins), etc. [2].
The escherichiosis causative agents are subdivided into two groups: diarrheagenic (DEC – diarrheagenic E. coli) and extraintestinal pathogenic (ExPEC – extraintestinal pathogenic E. coli). Five main diarrheagenic groups are relevant for cattle: enterotoxigenic (ETEC), enteropathogenic (EPEC), enterohemorrhagic (EHEC), Shiga toxin-producing (STEC) and necrotoxigenic (NTEC) E. coli [31].
Enterotoxigenic E. coli attach to the surface of enterocytes using fimbrial adhesins. The distinctive feature of the representatives of this pathogroup is presence of thermostable (stI and stII) and/or thermolabile (ltI and ltII) toxins that induce the secretion of electrolytes and water. This leads to diarrhea in infected animals and, as a result, to dehydration and death [2][32][33].
Despite the fact that EPEC less frequently cause GI disorders in calves than EHEC and ETEC, they require monitoring by the veterinarians due to their continuous on-farm circulation. The analysis of the frequency of occurrence of different E. coli pathogens in calves demonstrated that EPEC circulates almost twice as frequently in the healthy animals (14.6%) than in the diseased ones (7.5%) [4].
Enteropathogenic E. coli is characterized by the presence of the eae gene encoding the adhesive pathogenicity factor intimin and by the lack of ability to produce Shiga toxin (stx). Due to intimin, the bacterium attaches to enterocytes, after which they are rejected that further leads to diarrhea [4].
Based on the presence of the eae gene, Shiga toxic E. coli are divided into 2 groups: EHEC (STEC LEE+), which have the specified gene in their genome, and STEC (STEC LEE-), which lack it. The common features of the both groups include long-term persistence in the host, localization in the small intestine, and presence of genes encoding the ability to produce stx [2][32].
Based on the studies on the identification of various E. coli pathogens in calves conducted in 18 countries, it was found that STEC LEE+ is less common than STEC LEE–: in healthy calves, the frequency of their occurrence is 10.7 and 19.4%, respectively, and in the diseased ones – 6.0 and 18.2% [4].
Necrotoxigenic E. coli has a specific set of genes encoding cytotoxic necrotizing factor (CNF) and cytolethal distending toxin (CDT). This pathogroup has many properties of E. coli that cause diseases with extra-intestinal symptoms, such as presence of different fimbrial and afimbrial adhesins and the ability to resist the complement system [4][33].
Currently, there are two known types of cytotoxic necrotizing factors: CNF1 and CNF2. Presence of genes encoding the cytotoxic necrotizing factor 1 (CNF1) is more common in strains that cause diarrhea. The genes encoding the cytotoxic necrotizing factor 2 (CNF2) are found in E. coli, causing sepsis [33][34].
It should be noted that commensal E. coli, when interacting with pathogenic species, can acquire new genetic determinants encoding not only cell protective mechanisms, but also pathogenicity factors. Therefore, it is a mistake to classify a strain as pathogenic only on the basis of a serovariant, since there are E. coli that are included in the same serovar, but belong to different pathogroups and, as a result, cause different pathological processes. Such a tendency to variability may even lead to the acquisition of pathogenicity factors of different pathogens by one microorganism. For example, one of the publications mentions a hybrid strain containing EHEC and NTEC genes [31][35].
Infection of young animals with the colibacillosis causative agent occurs by the alimentary route. Infected adult animals play an important role in the E. coli spread, contaminating water, various indoor surfaces and bedding with bacteria, as a result of contact with which the infectious agent can get on the udder and later be transmitted to the calf [2].
In the beginning of the postnatal period, colibacillosis in calves takes more often an enteritic form and less often a septic form. The severe enteritic form is manifested by heavy diarrhea, rapid dehydration of the animal, sunken eyes, depression and exhaustion, dry and grayish skin. This disease form often ends with the death of the animal. When calves are housed in good sanitary conditions and the animal has colostral antibodies, the enteritis form may be mild [2].
Septic escherichiosis is caused by non-diarrheagenic E. coli (ExPEC). This form of colibacillosis develops due to untimely feeding with colostrum (primary) or in the presence of viral diseases (secondary). The septic disease clinical signs are expressed by ataxia, lameness, anorexia, hard breathing. The animal dies in 24–48 hours after their onset [2].
According to the scientific publications for the period from 1987 to 2021, the most escherichiosis-infected countries are Mexico, Brazil, India and Iran. The average global disease prevalence is 39.1% (Table 5). In Russia, the incidence rate is at the level of 30.2% (Table 6).
Table 5
Prevalence of bovine colibacillosis in the countries of the world
|
Country |
Region |
Estimated prevalence, % |
Source |
|
Australia (2011) |
Australia |
17.40 |
[6] |
|
Iran (2013) |
Asia |
86.70 |
[9] |
|
Iran (2010) |
76.45 |
[34] |
|
|
India (2009) |
75.00 |
[9] |
|
|
Pakistan (1997) |
54.00 |
[9] |
|
|
India (1993) |
23.00 |
[9] |
|
|
Germany (1997) |
Europe |
42.00 |
[9] |
|
Spain (2008) |
35.90 |
[9] |
|
|
Ukraine (2012–2019) |
31.68 |
[14] |
|
|
France (1999) |
20.30 |
[9] |
|
|
Sweden (1987–1988) |
11.50 |
[16] |
|
|
Sweden (1993) |
11.50 |
[9] |
|
|
Switzerland (2005–2006) |
5.50 |
[12] |
|
|
Brazil (2007) |
South and North America |
69.00 |
[20] |
|
Mexico (2000) |
63.70 |
[9] |
|
|
USA (2010–2011) |
1.80 |
[18] |
Table 6
Prevalence of bovine colibacillosis in the Russian Federation
|
Region of the Russian Federation |
Federal District |
Estimated prevalence, % |
Source |
|
Amur Oblast (2003–2005) |
Far Eastern |
33.00 |
[36] |
|
Amur Oblast (2016–2019) |
28.50 |
[37] |
|
|
Republic of Bashkortostan (2014–2016) |
Volga |
30.00 |
[38] |
|
Perm Krai (2010–2020) |
14.40 |
[38] |
|
|
Irkutsk Oblast (2004-2017) |
Siberian |
18.50 |
[39] |
|
Irkutsk Oblast (2001–2010) |
10.35 |
[38] |
|
|
Rostov Oblast (2021) |
Southern |
74.20 |
[40] |
|
Krasnodar Krai (1996–2015) |
43.55 |
[38] |
|
|
Rostov Oblast (2017) |
19.26 |
[41] |
It should be noted that E. coli causes diseases not only in calves, but also in adult animals, which, depending on the pathogen properties, can develop such infections as mastitis, metritis and endometritis. Tests of milk from mastitis-diseased animals demonstrated that the level of E. coli was four times higher than in the milk of healthy cows, which confirms the role of E. coli as one of the causative agents of bovine mastitis [42].
Currently, it has not been fully clarified whether any pathogenic E. coli strains cause mastitis, or whether the cause of infection are E. coli of some individual pathogroup. There is evidence that the mastitis causative agent is an individual pathogenic E. coli group – MPEC (mammary pathogenic E. coli), which includes many extra-intestinal pathogenic E. coli strains: when studying the genome of E. coli isolated from mastitis-diseased cows, the ExPEC-typical pathogenicity factors were identified. However, there is evidence of isolation of E. coli of STEC group from the diseased animals, which indicates its possible involvement in the disease onset [42][43].
Metritis and endometritis are also common E. coli-induced pathologies on the farms, which should not be underestimated, since under certain conditions they can lead to infertility and further culling of cows. Despite the fact that there is currently no clear opinion about the presence of a separate E. coli pathogroup that causes metritis and endometritis, some scientists identify six virulence genes, on the basis of which the E. coli strain that causes these pathologies can be presumably identified. kpsMTII gene is emphasized among them, which is probably responsible for the disease severity. Some publications note that presence of this gene carrying E. coli in the uterine microflora 9.2-fold reduces the probability of successful insemination [44].
MEASURES FOR PREVENTION OF VIRAL AND BACTERIAL DISEASES IN YOUNG CATTLE
All the above-mentioned data demonstrate that the issue of escherichiosis, rotavirus and coronavirus infections is quite acute for the Russian Federation and foreign countries and requires effective measures to solve it.
Vaccine prevention remains one of the most effective ways to control infectious diseases, including enteritis of viral and bacterial etiology, which is confirmed by publications of domestic and foreign authors.
For example, the study conducted in Canada in 2022 showed a two-fold decrease in the disease incidence in calves immunized with a live vaccine against coronavirus infection [45].
The results of immunization of pregnant cows with an inactivated vaccine against rotavirus and coronavirus infections on the infected farms of the Russian Federation, Belarus and Ukraine demonstrated that feeding newborn calves with colostrum and milk derived from vaccinated animals reduced the disease incidence in young animals 7 times and their mortality 6.4 times [46].
In Estonia, when studying the effect of the duration of feeding newborn calves from cows immunized with various inactivated vaccines against rotavirus, coronavirus infections and colibacillosis with colostrum and transitional milk, it was demonstrated that vaccination of pregnant animals reduced the mortality of young animals in comparison with the control groups. Feeding calves with colostrum and milk from vaccinated animals during the first 14 days after birth resulted in four-fold decrease in their mortality due to diarrhea. However, reduced period of colostrum and transitional milk feeding resulted in the decrease of the colostral immunity level in young animals [47].
In addition to preventing GI diseases, the vaccines with an optimal set of rotavirus, coronavirus and E. coli antigens can potentially reduce the number of pneumonia, mastitis and metritis cases in older animals on the farms.
When developing new products, it should be borne in mind that the effectiveness of specific preventive measures is influenced by such factors as the vaccine composition and the vaccination program implementation.
The first group of factors includes use of ineffective adjuvants or low-quality virus-containing source materials, as well as use of the active vaccine ingredient comprising strains with a low degree of antigenic matching with the field isolates circulating in a particular region. In this regard, monitoring of field isolates of rotavirus, coronavirus and pathogenic E. coli circulating in the Russian Federation and tracing changes in their genome will make it possible to timely adjust preventive measures and determine the most relevant vaccine composition when choosing from existing products or when developing new ones.
The effect of antigenic affinity of the pathogen vaccine strains and field isolates on the quality of vaccine prevention may indicate a greater effectiveness of the use of domestic products, since they are developed basing on the strains isolated in the Russian Federation.
The decrease in the effectiveness of immunization depends on the incorrect use of specific preventive measures: non-compliance with the dose, frequency and deadlines of vaccination; insufficient susceptible animal immunization coverage; non-compliance with the conditions of the vaccine storage and preparation for use; immunization of the diseased animals, etc.
CONCLUSION
Rotavirus-, coronavirus- and E. coli-induced infections are of great importance for animal husbandry, as they affect not only newborn calves (GI disorders, shortage of the replacement population, mortality of calves, treatment costs, etc.), but also adult cattle (mastitis, metritis, source of infection, etc.) thus resulting in significant economic losses.
The pathogens are widespread in many countries of the world. Herewith, the average incidence of rotavirus infection is 32.7%, coronavirus infection is 18.4%, and colibacilliosis is 39.1%.
The issue of the aforementioned infections does not lose its relevance for our country either. Thus, in Russia, the incidence of rotavirus and coronavirus infections exceeds the global average by 8.7 (41.4%) and 14.7% (33.1%), respectively. Of these diseases, escherichiosis is the least prevalent (30.2%), which may be due to significant seasonal temperature fluctuations in our country throughout the year and the active use of antibiotics on the Russian farms.
The wide variety and prevalence of the above-mentioned pathogens require an integrated approach to prevent infection of cattle (both young and adult animals); an adequate diet, practicing good hygiene, housing and feeding, implementation of animal quarantine measures, etc. are essential. When speaking of measures to prevent rotavirus, coronavirus infections and colibacilliosis, it should be mentioned that vaccination is one of the most effective ways to control them. Using a vaccine with an optimal set of antigens will not only protect calves from developing GI disorders and reduce their mortality, but also potentially reduce the number of pneumonia, mastitis and metritis cases in older animals on the farms, which makes the development of effective and safe vaccines against rotavirus, coronavirus infections and escherichiosis an urgent task.
References
1. Zeynalova Sh., Abbasov V. Infectious rotavirus and coronavirus of calves. Bulletin of Science and Practice. 2023; 9 (4): 167–172. https://doi.org/10.33619/2414-2948/89/22 (in Russ.)
2. Current infectious diseases of cattle: a manual. Ed. by prof. T. I. Aliper. Moscow: ZooVetKniga; 2021; 325–363; 624–630. (in Russ.)
3. Debelo M., Abdela H., Tesfaye A., Tiruneh A., Mekonnen G., Asefa Z., Moje N. Prevalence of bovine rotavirus and coronavirus in neonatal calves in dairy farms of Addis Ababa, Ethiopia: Preliminary study. BioMed Research International. 2021; 2021:5778455. https://doi.org/10.1155/2021/5778455
4. Kolenda R., Burdukiewicz M., Schierack P. A systematic review and meta-analysis of the epidemiology of pathogenic Escherichia coli of calves and the role of calves as reservoirs for human pathogenic E. coli. Frontiers in Cellular and Infection Microbiology. 2015; 5:23. https://doi.org/10.3389/fcimb.2015.00023
5. Batomunkuev A. S., Evdokimov P. I., Meltsov I. V. Rotaviral and coronaviral infections of cattle in the Irkutsk region. Veterinariya sel’skokhozyaistvennykh zhivotnykh. 2020; (5): 9–13. https://elibrary.ru/udcymq (in Russ.)
6. Izzo M. M., Kirkland P. D., Mohler V. L., Perkins N. R., Gunn A. A., House J. K. Prevalence of major enteric pathogensin Australian dairy calves with diarrhoea. Australian Veterinary Journal. 2011; 89 (5): 167–173. https://doi.org/10.1111/j.1751-0813.2011.00692.x
7. Swiatek D. L., Palombo E. A., Lee A., Coventry M. J., Britz M. L., Kirkwood C. D. Detection and analysis of bovine rotavirus strains circulating in Australian calves during 2004 and 2005. Veterinary Microbiology. 2010; 140 (1–2): 56–62. https://doi.org/10.1016/j.vetmic.2009.07.020
8. Qin Y.-F., Gong Q.-L., Zhang M., Sun Z.-Y., Wang W., Wei X.-Y., et al. Prevalence of bovine rotavirus among Bovidae in China during 1984–2021: A systematic review and meta-analysis. Microbial Pathogenesis. 2022; 169:105661. https://doi.org/10.1016/j.micpath.2022.105661
9. BashahunG. M., Amina A. Colibacillosisin calves: A review of literature. Journal ofAnimal Science and VeterinaryMedicine. 2017; 2 (3): 62–71. https://doi.org/10.31248/JASVM2017.041
10. Mayameei A., Mohammadi G., Yavari S., Afshari E., Omidi A. Evaluation of relationship between Rotavirus and Coronavirus infections with calf diarrhea by capture ELISA. Comparative Clinical Pathology. 2010; 19 (6): 553–557. https://doi.org/10.1007/s00580-009-0920-x
11. Gulliksen S. M., Jor E., Lie K. I., Hamnes I. S., Løken T., Åkerstedt J., Østerås O. Enteropathogens and risk factors for diarrhea in Norwegian dairy calves. Journal of Dairy Science. 2009; 92 (10): 5057–5066. https://doi.org/10.3168/jds.2009-2080
12. Lanz Uhde F., Kaufmann T., Sager H., Albini S., Zanoni R., Schelling E., Meylan M. Prevalence of four enteropathogens in the faeces of young diarrhoeic dairy calves in Switzerland. Veterinary Record. 2008; 163 (12): 362–366. https://doi.org/10.1136/vr.163.12.362
13. Dubanevich О. V., Tsiapsha Y. I. Viral pneumoenteritis of cattle in farms of the Republic of Belarus. Epizootology Immunobiology Pharmacology Sanitation. 2022; (2): 35–41. https://doi.org/10.47612/2224-168X-2022-2-35-41 (in Russ.)
14. Dubin R. A., Germanenko M. N. Viral-bacterial associations in calfs with gastrointestinal diseases. Epizootology Immunobiology Pharmacology Sanitation. 2020; (2): 21–29. https://elibrary.ru/jcdmgi (in Russ.)
15. Björkman C., Svensson C., Christensson B., deVerdier K. Cryptosporidium parvum and Giardia intestinalis in calf diarrhoea in Sweden. Acta Veterinaria Scandinavica. 2003; 44 (3–4): 145–152. https://doi.org/10.1186/1751-0147-44-145
16. Viring S., Olsson S.-O., Aleniús S., Emanuelsson U., Jacobsson S.-O., Larsson B., et al. Studies of enteric pathogens and γ-globulin levels of neonatal calvesin Sweden. Acta Veterinaria Scandinavica. 1993; 34 (3): 271–279. https://doi.org/10.1186/BF03548191
17. Garaicoechea L., Bok K., Jones L. R., CombessiesG., Odeon A., Fernandez F., Parreno V. Molecular characterization of bovine rotavirus circulating in beef and dairy herds in Argentina during a 10-year period (1994–2003). Veterinary Microbiology. 2006; 118 (1–2): 1–11. https://doi.org/10.1016/j.vetmic.2006.06.004
18. Cho Y.-I., Han J.-I., Wang C., Cooper V., Schwartz K., Engelken T., Yoon K.-J. Case-control study of microbiological etiology associated with calf diarrhea. VeterinaryMicrobiology. 2013; 166 (3–4): 375–385. https://doi.org/10.1016/j.vetmic.2013.07.001
19. Yurov K. P., Gulyukin M. I., Mnikova L. A., Alexeyenkova S. V., Ishkova T. A. Viruses causing frequent and emergent gastrointestinal infections of cattle (review). Veterinaria i kormlenie. 2021; (2): 55–58. https://doi.org/10.30917/ATT-VK-1814-9588-2021-2-15 (in Russ.)
20. Oliveira-Filho J. P., Silva D. P. G., Pacheco M. D., Mascarini L. M., Ribeiro M. G., Alfieri A. A., et al. Diarréia em bezerros da raça Nelore criados extensivamente: estudo clínico e etiológico = Diarrhea in Nelore calves: Clinical and etiologic study. Pesquisa Veterinária Brasileira. 2007; 27 (10): 419–424. https://www.researchgate.net/publication/262738825 (in Portuguese)
21. Kurbanmagomedov K. B. Etiology and the technological methods of the preventive maintenance enteritis of the calves of virus-bacterial etiology in the Republic of Daghestan. Veterinarnaya praktika. 2008; (4): 16–21. https://elibrary.ru/kdmwyx (in Russ.)
22. Batomunkuev A. S., Gretchenko Yu. A. Viral infectious diseases of cattle in the Irkutsk Region. East Siberian Journal of Biosciences. 2020; (101): 112–119. https://elibrary.ru/jlbqrm (in Russ.)
23. Parkhomenko Yu. S., Perepelkina I. S., Semenova E. V. Epizooticheskaya situatsiya v skotovodcheskikh khozyaistvakh Tsentral’nogo Chernozem’ya po rotavirusnoi i koronavirusnoi infektsiyam = Rotavirus and coronavirus infection situation on animal farms of the Central Black Earth Region. Biotekhnologiya v rastenievodstve, zhivotnovodstve i sel’skokhozyaistvennoi mikrobiologii: cbornik tezisov dokladov 19-i Vserossiiskoi konferentsii molodykh uchenykh, posvyashchennoi pamyati akademika RASKhN G. S. Muromtseva (Moskva, 15–16 aprelya 2019 g.) = Biotechnology in plant growing, animal breeding and agricultural microbiology: Proceedings of the 19th All-Russia conference of young researchers dedicated to the memory of G. S. Muromtsev, RAAS Academician (Moscow, 15–16 April 2019). Moscow: All-Russia Research Institute of Agricultural Biotechnology; 2019; 141–142. https://elibrary.ru/sbdxlo (in Russ.)
24. Skitovich G. S., Byadovskaya O. P., Prokhvatilova L. B. Tests of sera from cattle of different age groups for specific antibodies against rotaviruses and coronaviruses. Veterinary Science Today. 2013; (3): 45–47. https://elibrary.ru/ratowq
25. Mischenko V. A., Dumova V. V., Getmanskiy O. I., Kononov A. V., Ponomaryov A. P., Kukharkina О. V., et al. Koronavirusnaya infektsiya vzroslogo krupnogo rogatogo skota = Coronavirusinfection in adult cattle. Russian Journal of Veterinary Pathology. 2005; (3): 31–34. https://elibrary.ru/hsqdpf (in Russ.)
26. Kavalev M., Lamaka Yu. Coronavirus diseases of animals and vaccines against them. Science and Innovations. 2021; (8): 30–35. https://elibrary.ru/dvozcs (in Russ.)
27. Fukutomi T., Tsunemitsu H., Akashi H. Detection of bovine coronaviruses from adult cows with epizootic diarrhea and their antigenic and biological diversities. Archives of Virology. 1999; 144 (5): 997–1006. https://doi.org/10.1007/s007050050562
28. Mischenko V. A., Dumova V. V., Chernykh O. Yu., Kiselyov M. Yu., Mischenko A. V., Bakunov I. N., Kononov A. V. Bovine coronavirus distribution in ruminants. Veterinariya. 2010; (9): 18–21. https://elibrary.ru/mugzqp (in Russ.)
29. Nefedchenko A. V., Glotova T. I., Glotov A. G., Koteneva S. V., Terentieva T. E. Coronavirusinfection of calves on dairy complexes. Aktual’nye problemy lecheniya i profilaktiki boleznei molodnyaka: materialyMezhdunarodnoi nauchno-prakticheskoi konferentsii (Vitebsk, 2–4 noyabrya 2022 g.) = Current problems of young animal disease treatment and prevention: proceedings of the International research-to-practice conference (Vitebsk, 2–4 November 2022). Vitebsk: Vitebsk the Order of “the Badge of Honor” State Academy of Veterinary Medicine; 2022; 106–109. https://www.elibrary.ru/cipany (in Russ.)
30. Nechipurenko O. O. The dangerous E. coli and colibacillosis. Pigbreeding. 2019; (5): 35–37. https://elibrary.ru/oiobkr (in Russ.)
31. Fernández M., Casaux M. L., Fraga M., Vignoli R., Bado I., Zunino P., Umpiérrez A. Shiga toxin-producing Escherichia coli (STEC) associated with calf mortality in Uruguay. Microorganisms. 2023; 11 (7):1704. https://doi.org/10.3390/microorganisms11071704
32. Pobolelova Yu. I., Yatsentyuk S. P. Identification of patotypes and antibiotic resistance genes of museum strains of diarrheagenic E. coli. Trudi VIEV. 2018; 80 (1): 284–290. https://elibrary.ru/yqpalj (in Russ.)
33. Umpiérrez A., Ernst D., Fernández M., Oliver M., Casaux M. L., Caffarena R. D., et al. Virulence genes of Escherichia coli in diarrheic and healthy calves. Revista Argentina de Microbiología. 2021; 53 (1): 34–38. https://doi.org/10.1016/j.ram.2020.04.004
34. Shahrani M., Dehkordi F. S., Momtaz H. Characterization of Escherichia coli virulence genes, pathotypes and antibiotic resistance properties in diarrheic calves in Iran. Biological Research. 2014; 47:28. https://doi.org/10.1186/0717-6287-47-28
35. Zabrovskaia A. V. Pathogenic Escherichia coli: virulence factors, spread, diagnostic problems. International Bulletin of Veterinary Medicine. 2023; (4): 87–95. https://doi.org/10.52419/issn2072-2419.2023.4.87 (in Russ.)
36. Petrukhin M. A., Shulga N. N., Zhelyabovskaya D. A. Calves colibacillosisin the upper Amurregion. Bulletin of KSAU. 2012; (12): 113–116. https://elibrary.ru/pnfquz (in Russ.)
37. Gotskalo O. S. Analiz rasprostraneniya vozbuditelya kolibakterioza u sel’skokhozyaistvennykh zhivotnykh v Amurskoi oblasti = Analysis of colibacillosis agent spread in farm animals in Amur Oblast. Agropromyshlennyi kompleks: problemy i perspektivy razvitiya: materialy Vserossiiskoi nauchno-prakticheskoi konferentsii (Blagoveshchensk, 21 aprelya 2021 g.) = Agroindustrial complex: development challenges and prospects: proceedings of the All-Russia research-to-practice conference (Blagoveshchensk, 21 April 2021). Part 2. Blagoveshchensk: Far Eastern State Agrarian University; 2021; 46–51. https://elibrary.ru/nooxut (in Russ.)
38. Zhdanova I. N., MokrushinV. V., Kuznetsova M. V. Cattle colibacillosis in Perm krai: prevalence, sources of the causative agent and its biological characterization. Agricultural Biology. 2022; 57 (4): 776–790. https://doi.org/10.15389/agrobiology.2022.4.776eng
39. Batomunkuev A., Ablov A., Trofimov I., Dashko D., Lapa Y. Escherichiosis of farm animals in Irkutsk oblast. Vestnik of Buryat State Academy of Agriculture named after V. Philippov. 2018; (3): 47–53. https://elibrary.ru/yarftn (in Russ.)
40. Toropyno A. V., Shevchenko A. A., Shevchenko L. V. The role of cows in the distribution of pathogenic Escherichia to the offspring. Russian Journal of Veterinary Pathology. 2021; (1): 14–18. https://doi.org/10.25690/VETPAT.2021.16.54.007 (in Russ.)
41. Shevchenko A. A., Toropyno A. V. Epizoticalsituation of eserhihiosis in the Rostov region. Russian Journal of Veterinary Pathology. 2017; (3): 3–8. https://elibrary.ru/yngcws (in Russ.)
42. Murinda S. E., Ibekwe A. M., Rodriguez N. G., Quiroz K. L., Mujica A. P., Osmon K. Shiga toxin-producing Escherichia coli in mastitis: an international perspective. Foodborne Pathogens and Disease. 2019; 16 (4): 229–243. https://doi.org/10.1089/fpd.2018.2491
43. Jung D., Park S., Ruffini J., Dussault F., Dufour S., Ronholm J. Comparative genomic analysis of Escherichia coli isolates from cases of bovine clinical mastitis identifies nine specific pathotype marker genes. Microbial Genomics. 2021; 7 (7):000597. https://doi.org/10.1099/mgen.0.000597
44. Yamamura F., Sugiura T., Munby M., Shiokura Y., Murata R., Nakamura T., et al. Relationship between Escherichia coli virulence factors, notably kpsMTII, and symptoms of clinical metritis and endometritis in dairy cows. Journal of Veterinary Medical Science. 2022; 84 (3): 420–428. https://doi.org/10.1292/jvms.21-0586
45. Erickson N. E. N., April S., Campbell J. R., Homerosky E., Ware T., Dorin C., et al. Comparison of postweaning bovine respiratory disease treatment rates between non-vaccinated control beef calves and calves variably primed and boosted using commercially available bovine coronavirus vaccines. The Canadian Veterinary Journal. 2024; 65 (6): 581–586. https://pubmed.ncbi.nlm.nih.gov/38827595
46. Mischenko V. A., Getmanskyi O. I., Nikeshina T. B., Dumova V. V., Pavlov D. K., Zhbanova T. V., et al. Effectiveness of preventive vaccination against bovine neonatal viral diarrhea of rota- and coronavirus etiology. Proceedings ofthe FederalCentre forAnimalHealth. 2005; 3: 184–193. https://elibrary.ru/unwzbn (in Russ.)
47. Viidu D.-A., Mõtus K. Implementation of a pre-calving vaccination programme against rotavirus, coronavirus and enterotoxigenic Escherichia coli (F5) and association with dairy calf survival. BMC Veterinary Research. 2022; 18:59. https://doi.org/10.1186/s12917-022-03154-2
About the Authors
I. A. KruglovRussian Federation
Ilya A. Kruglov, Postgraduate Student, Leading Specialist, Laboratory for Biotechnologies and Viral Product Construction
Yur’evets, Vladimir 600901
A. V. Kononov
Russian Federation
Aleksandr V. Kononov, Dr. Sci. (Veterinary Medicine), Head of Laboratory for Biotechnologies and Viral Product Construction
Yur’evets, Vladimir 600901
A. A. Nesterov
Russian Federation
Alexander А. Nesterov, Cand. Sci. (Veterinary Medicine), Head of Sector, Laboratory for Biotechnologies and Viral Product Construction
Yur’evets, Vladimir 600901
S. V. Kononova
Russian Federation
Svetlana V. Kononova, Cand. Sci. (Biology), Leading Researcher, Reference Laboratory for Bovine Disease
Yur’evets, Vladimir 600901
O. V. Pruntova
Russian Federation
Olga V. Pruntova, Dr. Sci. (Biology), Professor, Chief Researcher, Information and Analysis Centre
Yur’evets, Vladimir 600901
Review
For citations:
Kruglov I.A., Kononov A.V., Nesterov A.A., Kononova S.V., Pruntova O.V. Role of rotavirus, coronavirus and Escherichia coli in disease etiology in young cattle (review). Veterinary Science Today. 2025;14(1):14-23. https://doi.org/10.29326/2304-196X-2025-14-1-14-23



































