Scroll to:
Use of a microbial supplement containing live bacteria Bacillus subtilis and their metabolites in dairy farming
https://doi.org/10.29326/2304-196X-2024-13-4-366-372
Abstract
The purpose of the research was to study parameters of immune status and milk yields in those cows that received a bacterial supplement containing live bacteria Bacillus subtilis strains B-239906 and B-249909 and their metabolites during a transition period. Animals in the experimental groups (10 animals in each) received the microbial supplement according to the following schemes: 14 days before calving (group 1), 14 days after calving (group 2), 14 days before and 14 after calving (group 3). Group 4 (n = 10) was a control one. On day 14 and day 28 after calving, the relative level of T-lymphocytes in blood of control cows and experimental group 2 did not significantly change compared with the level observed on day 1 after calving. While in groups 1 and 3, this indicator increased by 1.2–1.6 times throughout the whole experiment. In all cow groups, B-lymphocyte dynamics during the observation period was similar, i.e. an increase by day 14 and a decrease by day 28. The phagocytic activity of neutrophils in animals of all groups changed slightly. At the same time, the phagocytic index increased by 2.5–3.2 times throughout the experiment, which indicated an increase in nonspecific resistance of the body. Peak milk yields were recorded in cows of all experimental groups on day 90 of lactation. The maximum level (32.17 ± 3.33 kg) was observed in group 3. Within 150 days of observations, the average daily milk yields in animals of the experimental groups were: 24.50 ± 4.15 kg in group 1; 25.07 ± 4.38 kg in group 2; 25.33 ± 2.52 kg in group 3 and 22.75 ± 8.82 kg in the control group. The mass fraction of milk fat in all groups had no statistically significant differences throughout the entire observation period.
Keywords
For citations:
Oparina O.Yu., Krasnoperov A.S., Malkov S.V., Belousov A.I., Chernitskiy A.E., Vershinina I.Yu. Use of a microbial supplement containing live bacteria Bacillus subtilis and their metabolites in dairy farming. Veterinary Science Today. 2024;13(4):366-372. https://doi.org/10.29326/2304-196X-2024-13-4-366-372
INTRODUCTION
In recent years, the milk yield in Russian livestock holdings has exceeded the level of 8,000 kg of milk per year [1].
These achievements undoubtedly result from good breeding practice, appropriate conditions for realizing genetic potential of highly productive animals and introduction of physiologically and economically justified feeding schemes and control of zoohygienic parameters in animal housing [2][3]. The progress became possible because recent observations into digestion processes (at the molecular, cellular and body levels) and biological protein synthesis had been put into practice. In addition, great attention was paid to the principles of adequate nutrition, which take into account health status of cows and their milk yields [4][5].
When milk yield increases, vital functions often weaken: the immunity decreases and productive lifespan is reduced to 2–3 lactations. The animals are most likely removed from the herd due to metabolic disorders resulting from unbalanced diet in the pre-calving and post-calving periods [6][7].
Products of microbiological synthesis, their development and use, are considered as a solution to the existing problems in the livestock sector of the Russian Federation. They have proved to be effective in preventing diseases, reducing animal culling and improving digestibility of feed components. Their indirect effect is associated with an increase in milk yields, improved product quality, thus, enabling to provide people with safe food [8-10].
Live Bacillus subtilis bacteria and their metabolites that stimulate the growth of indigenous intestinal microbiota tend to be a promising component in new supplements. During their production, it is required to ensure proper conditions to maintain long-term stability during storage of the finished products [11-16].
The objective of the research was to study the effect of a microbial supplement containing live bacteria B. subtilis and their metabolites during the transition period on immunity status and milk yields in cows.
MATERIALS AND METHODS
The experiment was carried out with the support from the Department of Ecology and Non-Contagious animal pathogens of the Ural Scientific Research Veterinary Institute, a structural subdivision of the Ural Branch of the Russian Academy of Sciences. The work was done within the framework of the Basic Science Programme of the State Academies of Sciences in the field 4.2.1.5 “Development of technologies for lifetime quality management of livestock raw materials to obtain high-quality and safe food products”.
The study was conducted in Holstein cows (n = 40), at the age of 2–3 lactations, kept in one of the livestock holdings of the Sverdlovsk Oblast.
Four groups, each consisting of 10 animals, were selected for the experiment according to the principle of equivalents. When forming the groups, their physiological status, weight, age, nutrition level and milk yields from the previous lactation were taken into account.
The cows were kept in one typical cattle house, in a tie stall, and received a balanced feed. In addition to the basic diet, the experimental animals were administered 5 g of a new, domestically produced supplement, containing live bacteria B. subtilis strains B-239906 and B-249909 (at a concentration of 103 CFU/g of each species) and their metabolites. They received the supplement at different times: 14 days before calving (group 1), 14 days after calving (group 2), 14 days before and 14 days after calving (group 3); group 4 was a control one.
Clinical status and behavioural responses of the animals were daily examined. Starting from day 15 after calving, milk quality was assessed using CombiFoss FT+ (FOSS, Denmark) instrument. Milk yields were measured for 150 days.
Hematological tests were done three times: on day 1, 14 and 28 after calving with blood sampling taken from the tail vein.
Blood morphological composition was analyzed in Abacus Junior Vet analyser (Diatron, Austria) using standard reagents (Diatron, Austria). Leukocytic formula was calculated in blood smears stained by the Romanovsky – Giemsa method (300 cells per smear) using an Olympus BX 43 microscope (Olympus, Japan). Immunological blood tests included assessment of
T- and B-lymphocytes levels, T/B index, phagocytic index, phagocytic activity of neutrophils and monocytes using the method P. N. Smirnov et al. (2007)1. The reactions were observed in an Olympus BX 43 binocular microscope
(Olympus, Japan).
The animals were manipulated in compliance with the norms and ethical principles of the European Convention ETS No. 123.
Experimental data were processed in Excel (Microsoft, USA) and Statistica 10.0 (StatSoft Inc., USA) programmes, arithmetic means and standard deviations were determined. Reliability of differences was calculated using Student’s t-test (p ≤ 0.05).
RESULTS AND DISCUSSION
The analyzed blood parameters in experimental and control groups were within the reference range (Table 1). Hemoglobin, red blood cell volume, leukocyte, lymphocyte and platelet counts on days 14 and 28 post calving insignificantly varied and did not exceed the normal range. Changes in hematological parameters suggested normalization of haemopoiesis and restoration of immunobiological reactivity during the experiment, which did not contradict the works of other researchers [17].
Dynamics of neutrophils functional activity in cows during the experiment are given in Table 2.
It was found that on day 14 after calving, phagocytic activity and phagocytic index in all groups increased slightly, if compared to day 1, thus suggesting an increase in the absorption capacity of neutrophils. By day 28, phagocytic activity returned to the level reported during the first test, while the phagocytic index continued to increase. Thus, the parameter in cows increased by 2.5–3.2 times (p ≤ 0.01), if compared to day 1. The obtained data can be regarded as positive and suggesting an increased body resistance to negative factors and a reduced risk of inflammatory processes [10].
The absolute number of lymphocytes in all groups decreased in different ways during the post-calving period (Table 3). The most significant decrease was observed on day 28, i. e. by 40.5% (p ≤ 0.01) in group 1, if compared to day 1 after calving. In other groups, this parameter decreased by 19.4–20.8%, but did not exceed the reference range. Probably, these changes are associated with metabolic disorders and lack of energy after calving [4].
The relative content of T-lymphocytes in all groups on day 1 after calving ranged between 28.00 ± 4.64 and 36.20 ± 8.17%. On day 14 of observation, a 1.2-fold decrease was registered in the control group, and by day 28 it returned to the level of day 1. In animals of experimental groups 1 and 3, dynamical changes in the relative content of T-lymphocytes was opposite. Thus, throughout the whole observation period, this parameter increased by 1.2 (p ≤ 0.05) and 1.6 (p ≤ 0.01) times, respectively, which suggested the stimulation of cellular immunity.
The number of B-lymphocytes in experimental and control groups at the initial stage of the experiment (on day 1 after calving) ranged between 18.8 to 24.8%. During this period, a repeated pattern was revealed in all groups: an increase in B-lymphocyte synthesis by day 14 and a decrease by day 28.
Based on the above, we can assume that the dynamical changes in T- and B-lymphocytes levels in the post-calving period resulted from the indirect effect of the tested supplement and had a compensatory and restorative mechanism based on the regulation of intensity of biosynthetic processes, as was confirmed by a number of other researchers [4][18].
Positive effect of the tested supplement was reported when assessing the milk yield. Data on average milk yield per month and milk fat mass fraction are given in Figures 1 and 2.
Analyzing the milk yield, we found a positive variation in the average daily milk yields in animals that were given the supplement containing live B. subtilis bacteria and their metabolites as compared to cows from the control group. The results obtained suggested the animals were able to recover better after calving and were able to withstand long physical stress due to continuous milk secretion. Thus, by the end of the 3rd month of lactation, peak milk yields were registered in cows of all experimental groups compared to the first month: month 1 – 28.70 ± 5.92 kg, month 2 – 28.94 ± 6.84 kg, month 3 – 32.17 ± 3.33 kg. The opposite situation was observed in control animals – a decrease to the level of 27.90 ± 7.25 kg.
In the following months, a regular decrease in average daily milk yields was observed in cows of all groups, but within different ranges. For five months of observations, we obtained the following average daily milk yields: Experimental group 1 – 24.50 ± 4.15 kg, Experimental group 2 – 25.07 ± 4.38 kg, Experimental group 3 – 25.33 ± 2.52 kg, control group – 22.75 ± 8.82 kg.
An important criterion for assessing milk quality is the mass fraction of milk fat (Fig. 2).
The mass fraction of milk fat in all experimental groups increased by the end of the 2nd month of observation and was: month 1 – 3.63 ± 0.28 g/100 g, month 2 – 3.62 ± 0.31 g/100 g, month 3 – 3.77 ± 0.35 g/100 g (more by 7.1; 4.9 and 4.4%, respectively). These parameters in the control group had no significant differences.
In experimental animals, such changes were an indirect sign of body fat mobilization into milk. A significant increase in fat level during the 2nd month of lactation and its pronounced decrease during the 3rd month may be suggestive of a lactational exhaustion in cows of group 1. In the following months, insignificant variations of the parameters were observed with a return to average values.
Thus, the most pronounced effect was registered in group 3, where the diet was supplemented in the pre-calving and post-calving periods with the tested supplement containing live bacteria B. subtilis and their metabolites. The use of the supplement resulted in an increase in milk quantity and quality and the observed parameters complied with the results obtained by other authors [2][3][19][20].
Table 1
Hematological parameters in cows
|
Parameters |
Red blood cells, 1012/L |
Hemoglobin, g/L |
Thrombocytes, 109/L |
White blood cells, 109/L |
Lymphocytes, 109/L |
|
|
Standard* |
5.0–10 |
90–120 |
100–800 |
4.5–12 |
2.5–7.5 |
|
|
Control |
Day 1 |
6.41 ± 0.50 |
100.80 ± 4.44 |
259.60 ± 179.33 |
8.44 ± 1.66 |
4.89 ± 1.22 |
|
Day 14 |
6.75 ± 0.69 |
107.33 ± 3.51 |
284.00 ± 67.95 |
7.24 ± 0.76 |
4.26 ± 1.41 |
|
|
Day 28 |
6.30 ± 0.68 |
99.50 ± 7.05 |
265.60 ± 115.70 |
7.54 ± 2.03 |
3.94 ± 1.37 |
|
|
Experimental group 1 |
Day 1 |
7.05 ± 0.40 |
111.00 ± 6.16 |
327.20 ± 121.91 |
11.44 ± 3.14 |
6.13 ± 1.92 |
|
Day 14 |
6.87 ± 0.56 |
106.50 ± 8.89 |
426.00 ± 199.26* |
9.04 ± 3.10 |
5.17 ± 0.97 |
|
|
Day 28 |
6.05 ± 0.76 |
90.50 ± 6.36* |
348.30 ± 110.90 |
7.05 ± 2.06* |
3.65 ± 0.99* |
|
|
Experimental group 2 |
Day 1 |
7.49 ± 0.10 |
113.43 ± 7.28 |
207.71 ± 86.71 |
10.41 ± 2.44 |
5.52 ± 1.32 |
|
Day 14 |
7.12 ± 0.91 |
106.75 ± 9.78 |
405.25 ± 140.29* |
9.86 ± 5.06 |
4.84 ± 0.83 |
|
|
Day 28 |
6.74 ± 0.86 |
98.33 ± 10.02 |
310.70 ± 102.50 |
8.68 ± 0.70 |
4.37 ± 0.53 |
|
|
Experimental group 3 |
Day 1 |
6.67 ± 0.52 |
100.40 ± 10.64 |
301.40 ± 81.12 |
9.99 ± 2.15 |
5.26 ± 1.36 |
|
Day 14 |
6.94 ± 0.60 |
102.00 ± 5.79 |
349.60 ± 85.12 |
7.59 ± 1.23 |
4.71 ± 1.27 |
|
|
Day 28 |
6.20 ± 0.50 |
97.50 ± 2.38 |
305.60 ± 80.12 |
8.32 ± 0.55 |
4.17 ± 0.41 |
|
|
* differences are statistically significant at p < 0.05. |
||||||
Table 2
Functional activity of neutrophils in cows
|
Group of animals |
Parameters |
Time after calving |
||
|
Day 1 |
Day 14 |
Day 28 |
||
|
Control |
PhA, % |
37.60 ± 8.41 |
39.20 ± 8.41 |
36.00 ± 4.55 |
|
PhI, c. u. |
1.84 ± 0.19 |
2.31 ± 0.19 |
5.85 ± 0.17** |
|
|
Experimental group 1 |
PhA, % |
48.00 ± 8.37 |
52.60 ± 8.41 |
32.50 ± 0.71* |
|
PhI, c. u. |
1.96 ± 0.22 |
4.38 ± 0.19* |
6.25 ± 0.07** |
|
|
Experimental group 2 |
PhA, % |
42.29 ± 6.55 |
47.00 ± 8.41 |
34.00 ± 1.00 |
|
PhI, c. u. |
2.07 ± 0.37 |
2.62 ± 0.19 |
5.83 ± 0.64** |
|
|
Experimental group 3 |
PhA, % |
37.80 ± 9.76 |
42.80 ± 8.41 |
33.25 ± 7.45 |
|
PhI, c. u. |
2.36 ± 0.85 |
3.29 ± 0.19 |
5.92 ± 0.05** |
|
|
PhA – phagocytic activity; PhI – phagocytic index; * differences are statistically significant at p ≤ 0.05; ** differences are statistically significant at p ≤ 0.01. |
||||
Table 3
Indicators of cellular and humoral immunity in cows
|
Group of animals |
Time after calving |
Absolute number of lymphocytes, 109/L |
T-lymphocytes |
B-lymphocytes |
||
|
109/L |
% |
109/L |
% |
|||
|
Control |
Day 1 |
4.89 ± 1.22 |
1.42 ± 0.41 |
36.20 ± 8.17 |
0.96 ± 0.42 |
24.80 ± 8.67 |
|
Day 14 |
4.26 ± 1.41 |
0.65 ± 0.08* |
29.20 ± 3.32 |
0.68 ± 0.11 |
30.40 ± 5.48 |
|
|
Day 28 |
3.94 ± 1.37 |
1.60 ± 0.39 |
32.50 ± 6.61 |
1.21 ± 0.42 |
25.25 ± 10.21 |
|
|
Experimental group 1 |
Day 1 |
6.13 ± 1.92 |
1.62 ± 0.41 |
28.40 ± 8.02 |
1.08 ± 0.27 |
18.80 ± 2.68 |
|
Day 14 |
5.17 ± 0.97 |
1.60 ± 0.24 |
30.52 ± 2.62 |
1.36 ± 0.17 |
26.00 ± 7.01 |
|
|
Day 28 |
3.65 ± 0.99** |
1.56 ± 0.69 |
35.00 ± 2.83* |
1.09 ± 0.18 |
20.34 ± 5.66 |
|
|
Experimental group 2 |
Day 1 |
5.52 ± 1.32 |
2.03 ± 0.73 |
33.43 ± 6.73 |
1.31 ± 0.34 |
24.57 ± 8.56 |
|
Day 14 |
4.84 ± 0.83 |
1.56 ± 0.26 |
32.20 ± 2.82 |
1.27 ± 0.11 |
26.24 ± 6.31 |
|
|
Day 28 |
4.37 ± 0.53 |
1.64 ± 1.11 |
32.00 ± 18.52 |
0.79 ± 0.31 |
20.21 ± 4.51 |
|
|
Experimental group 3 |
Day 1 |
5.26 ± 1.36 |
1.26 ± 0.17 |
28.00 ± 4.64 |
0.88 ± 0.29 |
19.00 ± 5.92 |
|
Day 14 |
4.71 ± 1.27 |
1.78 ± 0.42 |
37.82 ± 2.44* |
1.20 ± 0.19 |
25.50 ± 5.23 |
|
|
Day 28 |
4.17 ± 0.41 |
2.11 ± 1.10* |
44.50 ± 15.86** |
0.88 ± 0.27 |
18.75 ± 2.50 |
|
|
* differences are statistically significant at p ≤ 0.05; ** differences are statistically significant at p ≤ 0.01. |
||||||
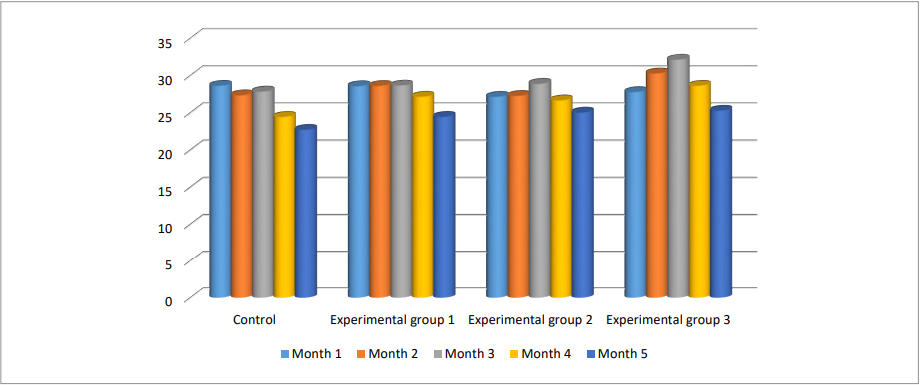
Fig. 1. Monthly dynamics of milk yields (kg)
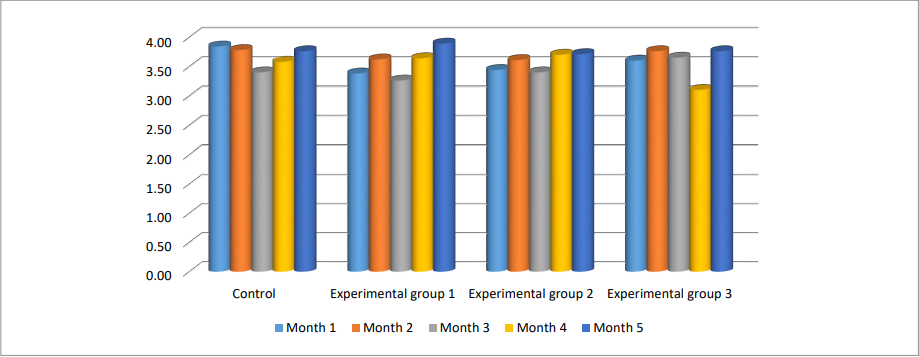
Fig. 2. Changes in the mass fraction of milk fat (g/100 g)
CONCLUSION
The use of the supplement containing live bacteria B. subtilis strains B-239906 and B-249909 and their metabolites had a positive effect on immunohematological parameters of cows’ blood. The revealed changes in immunity indicators (absolute number of lymphocytes,
T- and B-lymphocytes, phagocytic activity and phagocytic index) should be regarded as a compensatory-adaptation mechanism aimed at maintaining and normalizing metabolism.
Higher average daily milk yields were reported in cows of Experimental groups 1, 2 and 3, i. e. 24.50 ± 4.15; 25.07 ± 4.38 and 25.33 ± 2.52 kg of milk, respectively, compared to control group (22.75 ± 8.82 kg).
1. Panel of the most informative tests for assessment of animal resistance: methodological recommendations. Compiled by. P. N. Smirnov et al. Novosibirsk; 2007. 37 p. https://elibrary.ru/qkpwdx (in Russ.)
References
1. Doroshchuk S. V. Milk productivity and reproductive function of cows. Dostizheniya nauki i tekhniki APK. 2012; (11): 47–49. https://elibrary. ru/pizfoh (in Russ.)
2. Gumerov A. B., Belookov A. A., Loretz O. G., Gorelik O. V., Asenova B. K. The milk yield of cows when using probiotic enzyme preparations. Agrarian Bulletin of the Urals. 2018; (4): 5–9. https://elibrary.ru/xucoix (in Russ.)
3. Mikolaychik I. N., Morozova L. A., Abileva G. U., Subbotina N. A. Biological and productive factors of dry pregnant cows being fed with immunobiological additions. Vestnik Kurganskoj GSHA. 2016; (2): 44–47. https://elibrary.ru/wiqrgp (in Russ.)
4. Belousov A. I., Sokolova O. V., Bespamyatnykh E. N. The use of biochemical screening in assessing the productive health of high-yielding cows in the Sverdlovsk Region. Legal Regulation in Veterinary Medicine. 2018; (4): 278–280. https://doi.org/10.17238/issn2072-6023.2018.4.278 (in Russ.)
5. Eremenko V. N., Lytkin A. V., Mishagina I. V., Sinko O. V., Tyupenkova G. E., Luchinina I. G. Physiology of digestion and basis of rational nutrition. Proceedings of the Voronezh State University of Engineering Technologies. 2019; 81 (4): 159–165. https://doi.org/10.20914/2310-1202-2019-4-159-165 (in Russ.)
6. Shkuratova I. A., Ryaposova M. V., Sokolova O. V., Belousov A. I., Vereshchak N. A. Pathogenetic aspects of the development of the immune deficiency condition of the cattle in the industrial territories. Legal Regulation in Veterinary Medicine. 2018; (4): 255–258. https://doi.org/10.17238/issn2072-6023.2018.4.255 (in Russ.)
7. Shkuratova I. A., Belousov A. I., Krasnoperov A. S., Malkov S. V. Features of the biochemical profile of highly productive Holstein cows at primary ketosis. Veterinaria Kubani. 2022; (4): 7–9. https://doi.org/10.33861/20718020-2022-4-7-9 (in Russ.)
8. Zhukova Yu. S., Nagovitsyna E. V. Economic efficiency of application of probiotics in dairy cattle. Success of Modern Science and Education. 2017; 1 (5): 56–58. https://elibrary.ru/yrpslj (in Russ.)
9. Subbotina N. A., Morozova L. A., Mikolaychik I. N. Increasing the milk yield of cows on rations enriched by feed additive Megalac. Feeding of Agricultural Animals and Feed Production. 2016; (8): 39–46. https://elibrary.ru/wgxzvh (in Russ.)
10. Cheremushkina I. V., Shakhov A. G., Sashnina L. Yu., Chernitsky A. E., Yerina T. A. Antagonistic activity of a probiotic Prolam in point of bacterial pathogens and its influence on an intestines microbiocenosis, the immune and clinical status of calfs. Journal of Animal and Veterinary Advances. 2015; 14 (6): 182–191. https://doi.org/10.3923/javaa.2015.182-191
11. Vafin I. T., Shakirov Sh. K., Yusupova G. R., Volkov A. Ch. The effect of experimental probiotic supplements on milk production and milk quality of cows. Scientific notes Kazan Bauman State Academy of Veterinary Medicine. 2019; 238 (2): 42–45. https://doi.org/10.31588/2413-4201-1883-238-2-4246 (in Russ.)
12. Isupova M. V. Rezervy povysheniya molochnoi produktivnosti = Potential for increasing milk yields. Dairy and Beef Cattle Farming. 2020; (3): 39–40. https://elibrary.ru/fwotbl (in Russ.)
13. Koba I., Navruzshoeva G., Gorbatova Kh., Belkina Yu. S Batsell-M zdorovye korovy i kachestvennoe moloko = With BACELL-M: healthy cows and high-quality milk. Animal Husbandry of Russia. 2021; (12): 48–49. https://elibrary.ru/svrrpx (in Russ.)
14. Podobed L. I. Effectiveness of a probiotic based on lactic acid bacteria when changing diets in dairy cows. Agrarian science. 2020; (11–12): 15–16. https://doi.org/10.32634/0869-8155-2020-343-11-15-19 (in Russ.)
15. Ruin V. A., Kistina A. A., Prytkov Yu. N. The use of a probiotic complex in feeding dairy cows. Agrarian Scientific Journal. 2022; (4): 64–66. https://doi.org/10.28983/asj.y2022i4pp64-66 (in Russ.)
16. Malkov S. V., Krasnoperov A. S., Poryvaeva A. P., Oparina O. Yu., Belousov A. I., Brilliant A. N. Prospects for the use of a Bacillus subtilis metabolites-based feed additive in dairy farming. Veterinary Science Today. 2021; 10 (4): 342–348. https://doi.org/10.29326/2304-196X-2021-10-4-342-348
17. Rastorguyeva S. L., Ibishov D. F., Osipov A. P. Integrated effect of the Vitadaptin, the Guvitan-C, and the Germiviti on the absolute level of leukocytes, lymphocytes, and neutrophils in the peripheral blood of dry cows. Perm Agrarian Journal. 2019; (2): 136–142. https://elibrary.ru/udsipb (in Russ.)
18. Mityashova O. S., Gusev I. V., Lebedeva I. Yu. Metabolism and reproductive function during the postpartum period in first-calf cows when introducing the placenta extract. Agricultural Biology. 2017; 52 (2): 323–330. https://doi.org/10.15389/agrobiology.2017.2.323eng
19. Shatskikh E., Barmina I. The milk productivity of cows of Holstein black-and-white breed of American selection under conditions of Middle Urals. Head of Animal Breeding. 2016; (11): 3–8. https://elibrary.ru/wyxtlz (in Russ.)
20. Onoprienko N. A., Onoprienko V. V. Effect of probiotic preparation “Bacell-M” on milk productivity. Collection of Scientific Papers of North-Caucasus Research Institute of Animal Husbandry. 2016; 5 (1): 95–100. https://elibrary.ru/vwlqld (in Russ.)
About the Authors
O. Yu. OparinaRussian Federation
Olga Yu. Oparina, Cand. Sci. (Veterinary Medicine), Senior Researcher
112a Belinsky str., Ekaterinburg, 620142, Russia
A. S. Krasnoperov
Russian Federation
Alexander S. Krasnoperov, Cand. Sci. (Veterinary Medicine), Senior Researcher
112a Belinsky str., Ekaterinburg, 620142, Russia
S. V. Malkov
Russian Federation
Sergey V. Malkov, Cand. Sci. (Veterinary Medicine), Senior Researcher
112a Belinsky str., Ekaterinburg, 620142, Russia
A. I. Belousov
Russian Federation
Alexander I. Belousov, Dr. Sci. (Veterinary Medicine), Leading Researcher
112a Belinsky str., Ekaterinburg, 620142, Russia
A. E. Chernitskiy
Russian Federation
Anton E. Chernitskiy, Dr. Sci. (Biology), Leading Researcher
112a Belinsky str., Ekaterinburg, 620142, Russia
I. Yu. Vershinina
Russian Federation
Irina Yu. Vershinina, Researcher
112a Belinsky str., Ekaterinburg, 620142, Russia
Review
For citations:
Oparina O.Yu., Krasnoperov A.S., Malkov S.V., Belousov A.I., Chernitskiy A.E., Vershinina I.Yu. Use of a microbial supplement containing live bacteria Bacillus subtilis and their metabolites in dairy farming. Veterinary Science Today. 2024;13(4):366-372. https://doi.org/10.29326/2304-196X-2024-13-4-366-372



































