Scroll to:
Study of the vaccination effects against Staphylococcus aureus, causing mastitis and endometritis in cows
https://doi.org/10.29326/2304-196X-2024-13-4-360-365
Abstract
The high contagiousness of staphylococcal infections and emergence of antimicrobial resistant strains call for search and development of new highly effective drugs and vaccines against infectious animal diseases. Twenty adult pregnant black pied cows were used to form a test and a control groups (10 animals per group). The vaccine was administered twice subcutaneously in the middle third of the neck of the test animals: the first dose in a volume of 3 mL 55–70 days before calving, the second dose in the same volume 25–30 days before the expected calving. Control animals were injected subcutaneously with the same volume of sterile saline at the same dates. To evaluate the antigenicity of the vaccine against Staphylococcus aureus, blood was collected from animals of both groups: in the test group 14–16 days after booster vaccination, in the control group 14–16 days after second injection of the sterile saline. For bacteriological testing, milk samples from both groups were collected during the first month of lactation after calving. According to the results of serological testing, the antibody titer against Staphylococcus aureus in the test group ranged from 4.01 to 4.61 lg, its mean value was (4.34 ± 0.06) lg. In the test group, the mean antibody titers against Staphylococcus aureus were 5.8 times lower and were equal to (0.75 ± 0.09) lg with fluctuations from 0.3 to 1.2 lg. The bacteriological tests of milk in the control group revealed Staphylococcus aureus in 5 out of 10 samples, which is 50%. In the test group, the pathogen was detected in 20% of cases, which is 2.5 times lower than in the control group.
Keywords
For citations:
Ivanov E.V., Kapustin A.V., Avduevskaya N.N. Study of the vaccination effects against Staphylococcus aureus, causing mastitis and endometritis in cows. Veterinary Science Today. 2024;13(4):360-365. https://doi.org/10.29326/2304-196X-2024-13-4-360-365
INTRODUCTION
Staphylococcal diseases are the most frequent pathologies of animals and require highly qualified, long-term and expensive therapy [1][2]. Staphylococci can affect any tissue or organ and cause more than 100 different diseases: mastitis, endometritis, dermatitis, pneumonia, arthritis, purulent and wound infections, food poisoning, sepsis, etc. Enterotoxins, secreted by staphylococci in large quantities, have a complex effect on the animal immune system, resulting in its limited resistance. All staphylococcal enterotoxins are proteins with a relatively small molecular weight: from 26,900 to 29,600 Da. The classic staphylococcal enterotoxins comprise five main types: A, B, C, D and E (SEA-SEE), which are believed to be responsible for 95% of all staphylococcal poisoning cases. The prevention of food-borne diseases relies on the effectiveness of early diagnosis, that is, on the detection of enterotoxigenic staphylococci in milk, dairy and other food products [3][4].
The main causative agent of staphylococcosis in cattle is Staphylococcus aureus, which is isolated in 69.5% of staphylococcosis cases [5]. In biological samples collected from cows demonstrating signs of mastitis and endometritis, S. aureus bacteria are most frequent [6-8]. According to researchers, S. аureus was isolated from 8.8% of milk samples from healthy cows; in 59.3 to 62.8% of cases S. аureus was detected in milk samples from cows with subclinical mastitis; it was also found in udder secretions of mastitic cows and pooled milk in 28.8 and 18% of samples, respectively [6][9]. The bacteriological testing of vaginal swabs showed that one of the most frequent species responsible for postpartum endometritis was S. аureus (15.3% of the total tested cultures) [10].
The recovery rate from diseases caused by S. aureus is lower compared to other bacteria, which is explained by antimicrobial resistance and biofilm formation ability [11][12].
In recent years, S. аureus isolated from animals have become increasingly resistant to antimicrobials, including through the production of the enzyme β-lactamase, capable of cleaving penicillins and cephalosporins [13-17]. Staphylococcal mastitis caused by resistant strains of S. aureus is reported in almost 90% of large farms and commercial holdings where antibiotics are used [3][18-20]. Penicillin-resistant S. aureus are most common strains, which are identified as the first wave of resistance, and the second wave of resistance are methicillin-resistant S. aureus. Artemyeva O. A. et al. found that the highest resistance of S. aureus isolates in vitro was observed to erythromycin (82.5%) and fusidin (75.7%). Only seven isolated strains (6.8%) showed susceptibility to all tested antimicrobials, whereas 96 isolates were resistant to at least one of them [21]. According to I. S. Abdina et al., S. aureus antimicrobial resistance varied significantly, with the highest resistance reported to ampicillin (up to 57%), benzylpenicillin (up to 45%), doxycycline (up to 38%), oxacillin (up to 48%), streptomycin (up to 55%) and tetracycline (up to 45%) [22]. Other researchers established that of the 64 isolated S. aureus strains, 60 (93.7%) showed resistance to one or more antimicrobials. Multidrug resistance was observed in the tested S. aureus strains [11].
Despite the large increase in the incidence of staphylococcal infections in animals, effective drugs against them have not yet been developed. The spread of multidrug resistant S. aureus strains only complicates the disease control [23]. The successful treatment of animals with antibiotics brings risks of their consumption by humans. The high contagiousness of staphylococcal infections and the emergence of antimicrobial-resistant strains are the most serious problems and emerging threats for cattle industry, leading to decreased milk yields, impaired hygiene and quality of milk, increased costs for veterinary medicines and services, early culling of cows and their restricted performance. In this regard, it is necessary to search for alternative options based on staphylococci suppression, limiting the use of antimicrobials and minimizing their negative effects on the organism. One of such options is specific prevention, i.e. vaccines that provide reliable protection of animals from infectious diseases, contribute to the reduction in the use of antibiotics and prevent antimicrobial resistance of microorganisms [24][25]. Scientists have proven the effectiveness of the vaccines containing S. aureus antigen against mastitis and endometritis of cows. The studies revealed that immunization of animals against mastitis has a positive effect. After 6 months after first vaccination in the breeding facility and farm, the number of mastitis cases decreased by 16.6 and 7.3%, respectively, and somatic cell counts in the milk of high yielding cows decreased by 26.5 and 10.7%. The immunization remained effective even after 12 months post vaccination [26]. “Combovac-Endomast” vaccine (Vetbiochim, Russia) decreased the number of clinical mastitis cases by 7.8 times, of subclinical mastitis by 5.4 times, and endometritis by 3.7 times [27]. The experiments with the “Mastivac” vaccine in Laboratorios Ovejero S. A. Spain established that clinical mastitis cases decreased by 32% in the experimental group compared to the control one [9].
The aim was to study the effect of immunization against S. aureus, which causes mastitis and endometritis in cows.
MATERIALS AND METHODS
The study has been performed starting from 2021 in a family-operated farm in the Republic of Mordovia. Twenty adult pregnant black pied cows were used to form a test and control groups (10 animals per group). The animals were selected based on conformity principle, that is, all animals in both groups had standard parameters of the body weight, age, health status and management conditions. The vaccine was administered twice subcutaneously to the middle third of the neck of test animals. The first dose in a volume of 3 mL was administered 55–70 days before calving, the second dose of the same volume 25–30 days before the expected calving. In one immunizing dose the vaccine contains the following strains: Escherichia coli UR-10, Streptococcus agalactiae UR-7, Streptococcus dysgalactiae UR-16, Streptococcus uberis OB-5, Streptococcus pyogenes OB-4, Staphylococcus aureus OB-I4, Klebsiella pneumoniae K-2 (at least 3.5 × 109 CFU of each), inactivated with formalin (0.3% solution) and adsorbed on carbomer gel (10% by volume). The vaccine is intended to prevent mastitis and endometritis in cows. Control animals were injected subcutaneously with the same volume of sterile saline at the same dates.
Before the start of the experiment, milk and blood samples were collected from 20 cows with clinical mastitis, not included in any of the groups, for microbiological testing for S. aureus and serological testing for antibodies against S. aureus.
Blood was collected from animals of both groups to evaluate the antigenicity of the vaccine against S. aureus and milk samples for bacteriological testing. Blood sampling in the test group was performed 14–16 days after booster immunization, in the control group 14–16 days after second administration of sterile saline (placebo).
Animals were handled in compliance with the ethical standards adopted by the European Convention ETS No. 123.
The vaccine antigenicity was evaluated by the increase in the antibody titer against S. aureus measured by agglutination test. For the purposes of testing serum was diluted with sterile saline from 1:2 to 1:4096 and 0.5 mL of S. aureus OB-I4 antigen with concentration of ~5 × 108 CFU/mL was added to 0.5 mL of each serum dilution. The serum-antigen mixture was thoroughly mixed, placed in a thermostat and kept for 16–18 hours at (37 ± 1) °C, and then for another 3–4 hours at room temperature. After that, they were examined for agglutination occurrence. The results were interpreted with the agglutination viewer and a four-point visual scale was used: ++++ is 100% of cells agglutinated, complete liquid clearing; +++ means 75% of cells agglutinated, slight turbidity of the liquid; ++ means 50% of cells agglutinated, medium turbidity of the liquid; + is 25% of cells agglutinated, heavy turbidity of the liquid; – means no agglutination, homogeneous bacterial suspension.
Milk was pooled from all four udder lobes, but milk samples from different animals were tested individually, that is, they were not pooled with each other. The bacteriological testing of milk was performed during the first month of lactation after calving in accordance with the “Guidelines for the bacteriological testing of milk and udder secretion of cows”1. S. aureus was identified in accordance with GOST 30347-2016 “Milk and milk products. Methods for determination of Staphylococcus aureus”2, and by mass spectrometry (MALDI-ToF)3.
The results were statistically processed using generally accepted methods with Microsoft Office Excel 2010, Stat Plus 2009 software.
RESULTS AND DISCUSSION
According to the results of serological testing, the antibody titer against S. aureus in the test group ranged from 4.01 to 4.61 lg, its mean value was (4.34 ± 0.06) lg. In the test group, the mean antibody titers against S. aureus were 5.8 times lower and were equal to (0.75 ± 0.09) lg with fluctuations from 0.3 to 1.2 lg (Table).
As the data obtained show, the immunization facilitated the increase in antibodies against S. aureus in cows of the test group, which confirms the high potency of the vaccine. In addition, the titer of antibodies to S. aureus in the blood of animals during preliminary serological testing was almost identical to the titer in the control group and was equal to (0.70 ± 0.05) lg.
Loskutova I. V. et al. also found that clinically healthy cows immunized with the Mastivac vaccine (Laboratorios Ovejero S. A., Spain) containing S. aureus induced antibodies to S. aureus enterotoxins which is evident of the vaccine ability to induce an immune response in animals against the bacterium [12]. Hadimli H. H. et al. evaluated the effectiveness of the staphylococcal vaccine on humoral immunity against S. aureus in vaccinated animals [28].
At the next stage, a bacteriological testing of milk for S. aureus was performed.
During a preliminary testing of milk from 20 cows with clinical mastitis, S. aureus was found in 11 samples, which amounted to 55% of the total number of samples tested. In the control group, S. aureus was isolated in half of the milk samples tested (the bacterium was detected in 5 out of 10 samples (50%). In the milk of cows from the test group, S. aureus was found in 20% of samples (2 samples), which is 2.7 and 2.5 times lower compared to the group of cows before the experiment and the control group, respectively.
The figure shows that S. aureus isolation rate from non-vaccinated animals (the control group and the group of cows with clinical mastitis) is almost the same; in immunized cows, it was significantly lower.
The effectiveness of S. aureus vaccination against mastitis was also established by other researchers, who isolated the bacterium from 73.3% of milk samples before vaccination, and 6 months after the first immunization the isolation rate decreased to 26.6%, that is, by 2.7 times [29].
Table
S. aureus antibody titer in blood of cows from test and control groups
|
Microorganism |
Antibody titer |
|
|
test group (10 animals), lg |
control group (10 animals), lg |
|
|
S. aureus |
4.31 |
0.6 |
|
4.01 |
0.3 |
|
|
4.61 |
1.2 |
|
|
4.01 |
0.3 |
|
|
4.61 |
0.9 |
|
|
4.01 |
0.6 |
|
|
4.31 |
1.2 |
|
|
4.31 |
0.3 |
|
|
4.61 |
0.9 |
|
|
4.61 |
1.2 |
|
|
М ± m |
4.34 ± 0.06 |
0.75 ± 0.09 |
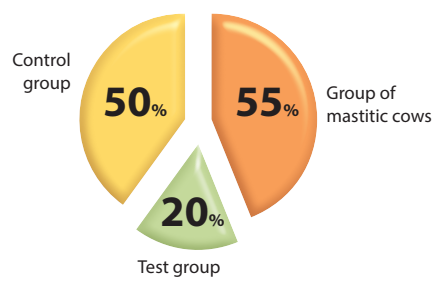
Fig. S. aureus isolation rate in the tested milk samples
CONCLUSION
It was found that double vaccination facilitated the increase in antibodies levels against S. aureus; the mean titers in the test group were 5.8 times higher than in the control group. In milk samples collected from vaccinated animals, the S. aureus isolation rate decreased by 2.7 and 2.5 times compared with groups of non-immunized animals. The results obtained show that the vaccination induces immune response in animals against S. aureus.
1. Guidelines for the bacteriological testing of milk and udder secretion of cows: approved by Chief Veterinary Department under the USSR Ministry of Agriculture No. 115-69 on 30.12.1983. https://base.garant.ru/72125912
2. GOST 30347-2016 Milk and milk products. Methods for determination of Staphylococcus aureus. https://docs.cntd.ru/document/1200142424
3. Guidelines for microorganism identification using MALDI Biotyper mass spectrometer when testing food raw materials and food products (approved by the Rosselkhoznadzor Scientific Technical Commitees on 03.04.2014).
References
1. Evglevsky D. A., Zherebilov N. N., Tagirmirzoev B. M., Steblovsky E. A. Biologicheskie svoistva stafilokokkov i povyshenie spetsificheskoi i antibakterial’noi profilaktiki i terapii boleznei zhivotnykh = Biological properties of staphilococci and improvement of specific and antibacterial prevention and therapy of animal diseases. Vestnik Kurskoj Gosudarstvennoj Sel’skoho-Zyajstvennoj Akademii. 2013; (9): 70–71. https://elibrary.ru/rsblmf (in Russ.)
2. Chuprunov V. P., Surovtsev V. I., Fjodorov T. E., Gusev V. V. Novyi fermentnyi preparat vetlizostafin dlya lecheniya stafilokokkozov zhivotnykh = Novel enzymatic drug vetlyzostaphin to treat animal staphilococcoses. Russian Journal of Veterinary Pathology. 2003; (2): 40–41. https://elibrary.ru/hsobpj (in Russ.)
3. Popov P. A., Osipova I. S. Study of immuno-enzyme diagnostics for Staphylococcus aureus enterotoxin based on hyperimmune sera. Polythematic online scientific journal of Kuban State Agrarian University. 2020; (161). https://doi.org/10.21515/1990-4665-161-008 (in Russ.)
4. Andryushchenko I. A., Gupal D. A., Degtyar A. S. Vliyanie mastita na molochnuyu produktivnost’korov = Effects of mastitis on milk performance in cows. Topical Issues of Modern Science: Theory, Technology, Methodology and Practice: Theory, Technology, Methodology and Practice: Collection of scientific articles based on proceedings of VII International Scientific and Practical Conference (Ufa, 14 December 2021). Ufa: NITs Vestnik nauki; 2021; 89–92. https://elibrary.ru/qsxqey (in Russ.)
5. Ablov A. M., Anganova E. V., Batomunkuev A. S. The staphylococcosis of animals and birds in the Baikal region. Vestnik of Omsk SAU. 2014; (3): 22–27. https://elibrary.ru/tflvnf (in Russ.)
6. Muzyka V. P., Stetsko T. I., Pashkovskaya M. V., Padovsky V. N. Monitoring chuvstvitel’nosti stafilokokkov k antimikrobnym veshchestvam = Monitoring of staphylococcus susceptibility to antimicrobials. Transactions of the Educational Establishment “Vitebsk the Order of “the Badge of Honor” State Academy of Veterinary Medicine”. 2012; 48 (2-1): 119–122. https://elibrary.ru/senxvf (in Russ.)
7. Polegaeva K. S., Rodin M. I., Sedov A. V., Gorbacheva Ju. A., Yakimov V. V. Species composition of microorganisms in inflammation of the uterine mucosa in cows. Vestnik ASAU. 2023; (2): 11–20. https://elibrary.ru/ehjizh (in Russ.)
8. Levchenko A. Effect of in vitro on Tseftioklynu Staphylococcus aureus isolated from milk cows mastytnyh. Scientific Messenger of Lviv National University of Veterinary Medicine and Biotechnologies named after S. Z. Gzhytskyj. 2014; 16 (3-1): 212–215. https://elibrary.ru/vlqwld (in Ukrainian)
9. Varenikov M. V., Tashlanov V. V., Morozov I. A. Mastitis prophylaxis leads to high profitability of milk production. Dairy and Beef Cattle Farming. 2014; (8): 32–35. https://elibrary.ru/tecawh (in Russ.)
10. Ivanyuk V. P., Bobkova G. N. Etiopathogenesis of postpartum endometritis in cows. Izvestia Orenburg State Agrarian University. 2022; (2): 191–195. https://doi.org/10.37670/2073-0853-2022-94-2-191-195 (in Russ.)
11. Rychshanova R. M., Mendybayeva A. M., Mukanov G. B., Shevchenko P. V., Bermukhametov Zh. Zh. Ustoichivost’ k antibiotikam i sposobnost’ k obrazovaniyu bioplenok zolotistogo stafilokokka, vydelennogo iz moloka korov Kostanaiskoi oblasti RK = Antimicrobial resistance and ability of biofilm formation of Staphilococcus aureus, isolated from cow milk in Kostanayskaya Oblast of the Republic of Kazakhstan. Innovations and Food Safety. 2021; (3): 29–39. https://doi.org/10.31677/2072-6724-2021-33-3-29-39 (in Russ.)
12. Loskutova I. V., Shchannikova M. P., Fursova K. K., Shepelyakovskaya A. O., Artemyeva O. A., Nikanova D. A., et al. Determination of specific antibodies to enterotoxins of Staphylococcus aureus in blood and colostrum from cows. Agricultural Biology. 2017; 52 (6): 1273–1278. https://doi.org/10.15389/agrobiology.2017.6.1273eng (in Russ.)
13. Karelin A. K. K voprosu o konkurentsii mezhdu plesnevymi gribami roda penitsill i shtammami zolotistogo stafilokokka, imeyushchimi ustoichivost’ k penitsillinu = Digging deeper into competition between Penicillium mold fungi and penicillin-resistant Staphilococcus aureus strains. Mechnikovskie chteniya-2022: materialy 95-i Vserossiiskoi nauchno-prakticheskoi studencheskoi konferentsii s mezhdunarodnym uchastiem (Sankt-Peterburg, 28 aprelya 2022 g.) = Mechnikov readings-2022: Proceedings of 95th All-Russian Scientific and Practical Students Conference with International Participation (Saint Petersburg, 28 April 2022). Saint Petersburg: North-Western State Medical University named after I. I. Mechnikov; 2022; 245. https://elibrary.ru/nlubps (in Russ.)
14. Terletskiy V. P., Tyshchenko V. I., Novikova O. B., Gaplaev M. Sh. Identifikatsiya patogennykh bakterial’nykh shtammov zolotistogo stafilokokka v sovremennoi profilakticheskoi veterinarii = Identification of pathogenic Staphylococcus aureus strains in modern preventive veterinary medicine. Effectivnoe zhivotnovodstvo. 2019; (2): 50–52. https://elibrary.ru/zamvud
15. Torutanov P. S. A comparison of the effectiveness of the peptides and antibiotics against Staphylococcus aureus. Ustoichivoe razvitie nauki i obrazovaniya. 2020; (5): 216–221. https://elibrary.ru/qgecrd (in Russ.)
16. Gostev V. V., Sidorenko S. V. Methicillin-resistant Staphylococcus aureus: the problem of expantion in the world and in Russia. Farmateka. 2015; (6): 30–38. https://elibrary.ru/tqaneh (in Russ.)
17. Dmitrenko O. A. Staphylococcus aureus toxins and toxoids: role in pathogenesis and prevention of staphylococcal infections. Molecular medicine. 2016; 14 (4): 10–19. https://elibrary.ru/wfqkpb (in Russ.)
18. Aziamov M. A. The partial replacement of antibiotics with biologically active substances at treatment of cows’ mastitis. Theoretical and Applied Ecology. 2018; (4): 127–134. https://doi.org/10.25750/1995-4301-2018-4127-134 (in Russ.)
19. Avduevskaya N. N. Staphylococcus aureus is one of the main pathogens of mastitis of lactating cows. Russian Journal “Problems of Veterinary Sanitation, Hygiene and Ecology”. 2020; (2): 245–249. https://doi.org/10.36871/vet.san.hyg.ecol.202002020 (in Russ.)
20. Avduevskaya N. N. Sensitivity of Staphylococcus aureus isolated from the udder of cows sick with mastitis, to complex preparations of antimicrobial action. Russian Journal “Problems of Veterinary Sanitation, Hygiene and Ecology”. 2017; (3): 56–59. https://elibrary.ru/zhtuoj (in Russ.)
21. Artemeva O. A., Nikanova D. A., Kolodina E. N., Romanova V. V., Brovko F. A., Zinovieva N. A. Phenotypic resistance to antibiotics of Staphylococcus aureus strains isolated from cow milk. Agricultural Biology. 2019; 54 (6): 1257–1266. https://doi.org/10.15389/agrobiology.2019.6.1257eng
22. Abdina I. S., Rakhmanova A. V., Shurygina M. N., Lykov I. N. Sensitivity to staphylococcus antibiotics, isolated from various environments. Vestnik Kaluga State University. 2023; (2): 61–65. https://elibrary.ru/kqrlgs (in Russ.)
23. Abdraimova N. K., Kornienko M. A., Bespiatykh D. A., Gorodnichev R. B., Shitikov E. A. Sovmestnoe primenenie bakteriofaga vB_SauM515A1 i antibiotikov protiv shtammov Staphylococcus aureus s mnozhestvennoi lekarstvennoi ustoichivost’yu = Combined use of vB_SauM-515A1 bacteriophage and antibiotics against multi-drug resistant Staphylococcus aureus. VIII Pushchinskaya konferentsiya «Biokhimiya, fiziologiya i biosfernaya rol’ mikroorganizmov»; Shkola-konferentsiya molodykh uchenykh, aspirantov i studentov «Geneticheskie tekhnologii v mikrobiologii i mikrobnoe raznoobrazie»: sbornik tezisov = VIII Puschino Conference “Biochemistry, Physiology and Biospheric Role of Microorganisms”; Conference and workshop for early career scientists, postgraduate students and students “Genetic Technologies in Microbiology and Microbial Diversity”: collection of abstracts. Moscow: GEOS; 2022; 102–104. https://doi.org/10.34756/GEOS.2022.17.38305 (in Russ.)
24. Vaktsinatsii STARTVAC® – eto vygodnye investitsii dlya profilaktiki mastita = STARTVAC® vaccinations are value-enhancing investments for mastitis prevention. Sfera: Tekhnologii. Korma. Veterinariya. 2017; 1 (4): 45–47. https://elibrary.ru/zmrbxl (in Russ.)
25. Piepers S. Vaccination cows against mastitis: an overview. Veterinariya. 2018; (11): 10–13. https://elibrary.ru/ymhvzj (in Russ.)
26. Isakova M. N., Ryaposova M. V., Sivkova U. V. The effectiveness of the use of mastitis vaccine in breeding farms. Legal Regulation in Veterinary Medicine. 2023; (1): 51–55. https://doi.org/10.52419/issn2782-6252.2023.1.51 (in Russ.)
27. Ivanov E. V., Kapustin A. V., Laishevtsev A. I., Supova A. V., Aliper T. I., Verkhovsky O. A. The effectiveness of the Kombovak-Endomast vaccine in the fight against infectious mastitis and endometritis in cows. Veterinariya. 2023; (11): 10–13. https://doi.org/10.30896/0042-4846.2023.26.11.10-13 (in Russ.)
28. Hadimli H. H., Erganis O., Kav K., Sayin Z. Evaluation of a combined vaccine against staphylococcal mastitis in ewes. Bulletin of the Veterinary Institute in Pulawy.2005; 49 (2): 179–182. https://jvetres.piwet.pulawy.pl/files/archive/20052/20052179182.pdf
29. Klimova L. A., Riaposova M. V., Shkuratova I. A., Tarasenko M. N., Tarasov M., Pavlova N. A. Experience of Startvac use on LLC “Nekrasovo-1” against bovine mastitis, Sverdlovsk Region. Veterinariya. 2014; (9): 34–37. https://elibrary.ru/slpkxh (in Russ.)
About the Authors
E. V. IvanovRussian Federation
Evgeny V. Ivanov, Cand. Sci. (Biology), Leading Researcher, Laboratory of Microbiology
24/1 Ryazansky prospekt, Moscow 109428, Russia
A. V. Kapustin
Russian Federation
Andrey V. Kapustin, Dr. Sci. (Biology), Associate Professor, Deputy Director for Scientific Work
24/1 Ryazansky prospekt, Moscow 109428, Russia
N. N. Avduevskaya
Russian Federation
Natalia N. Avduevskaya, Cand. Sci. (Biology), Researcher
10 Chekhov str., Vologda 160009, Russia
Review
For citations:
Ivanov E.V., Kapustin A.V., Avduevskaya N.N. Study of the vaccination effects against Staphylococcus aureus, causing mastitis and endometritis in cows. Veterinary Science Today. 2024;13(4):360-365. https://doi.org/10.29326/2304-196X-2024-13-4-360-365
JATS XML



































