Scroll to:
Canine adenovirus serotype 2 isolation and determination of its cultivation parameters
https://doi.org/10.29326/2304-196X-2024-13-4-352-359
Abstract
Adenovirus infection in dogs caused by canine adenovirus serotype 2 predominantly results in respiratory disease typically manifested by respiratory tract lesions. Infectious laryngotracheitis is the most often recorded in dogs in the central part of the Russian Federation and its incidence tends to increase. Therefore, preventive immunization against this disease remains important. Primarily, the virus strains currently important and circulating in the particular territory shall be used for vaccine production to induce long-term and strong immunity in animals. The study was aimed at isolation of canine adenovirus type 2 remaining stable during five or more passages from the biological samples collected from animals with adenovirus infection signs as well as at determination of its cultivation parameters. As a result, five virus isolates were recovered, one of the recovered virus isolates had optimal properties for its use for vaccine production. Comparative analysis of continuous Vero, MDCK (NBL-2 and NBL-9 line) cell cultures as well as primarily trypsinized cell cultures (baby dog kidney, baby dog spleen, baby cat kidney, baby cat spleen) for their susceptibility to the recovered virus showed that MDCK (NBL-2 line) was the most susceptible. The virus cultivation parameters in this cell culture was determined at the next step. The following optimal conditions under which the virus accumulated to the maximum titres were determined: cell culture monolayer age for inoculation – 48 hours, multiplicity of infection – 0.01 TCID50/cell, preliminary holding time – 60 min, temperature – (37.0 ± 0.5) °С, cultivation period – 120 hours.
Keywords
For citations:
Klimova A.A. Canine adenovirus serotype 2 isolation and determination of its cultivation parameters. Veterinary Science Today. 2024;13(4):352-359. https://doi.org/10.29326/2304-196X-2024-13-4-352-359
INTRODUCTION
Adenovirus infection in dogs (infectious laryngotracheitis) caused by adenovirus serotype 2 (CAV-2) predominantly results in respiratory disease typically manifested by respiratory tract lesions [1-3]. The infection agent is widespread in the central part of the Russian Federation due to the dense population of dogs and regular events characterized by crowded animal keeping. Despite regular preventive vaccination of animals, infectious laryngotracheitis incidence tends to increase.
The canine adenovirus serotype 2 belongs to Varidnaviria realm, Bamfordvirae kingdom, Preplasmiviricota phylum, Tectiliviricetes class, Rowavirales order, Adenoviridae family, Mastadenovirus genus, Mastadenovirus canidae species. It was included in the register of the International Committee on Taxonomy of Viruses (ICTV) in 1976 [4]. The virus was first isolated in Canada in 1961 [5].
Currently, two serotypes of the causative agent of adenovirus infection in dogs are known: the 1st serotype of the virus (Canine adenovirus 1, CAV-1) is characterized by its systemic effect on the animal’s body, affects most of the main organs and causes infectious hepatitis; the 2nd serotype of the virus (Canine adenovirus 2, CAV-2), like many representatives of the Mastadenovirus genus, is characterized by local effect and causes mainly respiratory tract lesions, less often gastrointestinal tract lesions [2][6-9].
Canine adenovirus serotype 1 (CAV-1) is more virulent than CAV-2. Canine adenovirus serotype 2 (CAV-2) is a nonenveloped DNA virus, weakened variant of CAV-1, sharing approximately 75% nucleotide sequence identity [10]. Coinfection with other viruses increases the pathogenicity of adenoviruses [11].
Canine adenovirus serotype 2 has been reported in dogs, raccoons, horses, cattle, cats and wolves. It shows subclinical circulation in the population of wild carnivores [11-14].
It should be noted that canine infectious respiratory disease complex called infectious laryngotracheitis in dogs, or kennel cough, is caused by several different microorganisms, that, besides adenovirus serotype 2, include the following pathogens: canine distemper virus, canine herpesvirus, canine parainfluenza virus, canine influenza virus, canine respiratory coronavirus, canine pneumovirus, as well as the following bacteria: Mycoplasma cynos, Bordetella bronchiseptica and Streptococcus equi subspecies [3][15-18]. Bocavirus and canine hepacivirus rarely cause respiratory disease symptoms and are not considered during differentiation [19].
Various data on CAV-2 cultivation are available. Adenoviruses are known to demonstrate the best growth in the cells of their natural animal hosts [6]. Dog kidney cell cultures are the most susceptible to the virus but other types of canine cell cultures are not susceptible to the virus. Primary or continuous other mammalian cell cultures, such as humans, sheep, and monkeys, are also not susceptible to the virus. In world practice, Madin – Darby canine kidney (MDCK) cell culture is considered optimal for CAV-2 cultivation [11][20-22]. According to other studies, CAV-2 can be also cultivated in Vero cell culture (continuous African green monkey kidney cell culture) [7].
The study was aimed at isolation of canine adenovirus serotype 2 remaining stable during five or more passages, as well as at determination of its cultivation parameters.
Cell cultures for CAV-2 virus reproduction were selected by serial passaging from available cell lines mainly used for cultivation of canine viruses and recommended for the cultivation of adenoviruses.
Primary cell line derived from naturally susceptible animals that are more susceptible to the infection is commonly used for virus isolation. Therefore, primary trypsinized baby dog kidney cell culture was selected for the first passage. Also, this cell culture was of priority for the virus isolation from pathological materials owing to high embryonic cell potential for growth.
MATERIALS AND METHODS
Biological samples (nasal and oral swabs) were collected from dogs suspected to have adenovirus infection, that were brought to veterinary clinics and shelters in the Vladimir, Vologda and Nizhny Novgorod Oblasts in 2019–2022.
Diagnosis. “ADENOVIR” commercial test system Central Research Institute for Epidemiology of the Rospotrebnadzor, Russia) was used to confirm CAV-2 presence in samples and to carry out differential diagnosis using polymerase chain reaction (PCR).
During CAV-2 virus passaging Asan Easy Test CAV2 immunochromatographic test system (Asan Pharmaceutical Co., Ltd., Korea) was used for the virus antigen detection according to the manufacturer’s instructions.
Cell cultures. The following cell cultures were selected for testing: continuous MDCK cell cultures (NBL-2 and NBL-9 lines) and Vero cell culture; primary trypsinized baby dog kidney, baby dog spleen, baby cat kidney and baby cat spleen cell cultures. The initial cell concentration in the cell suspension for MDCK NBL-2 and NBL-9 lines was 400 ths cells/cm3, for Vero cell culture – 200–250 ths cells/cm3, for primary trypsinized cell cultures – 300–400 ths cells/cm3. Completely formed monolayer without cell degeneration signs was a criterion for cell culture selection for inoculation. These cultures were subjected to stationary cultivation at a temperature of (37.0 ± 0.5) °C.
Plastic cell culture flasks (T25) with surface area 25 cm3 were used for passaging.
Nutrient media. The following nutrient media indicated in the cell culture data sheets were used: semi-synthetic nutrient medium supplemented by 5% bovine serum, antibiotics (streptomycin 100 μg/cm3 and penicillin 100 U/cm3) was used as growth medium for and serum-free semi-synthetic nutrient medium was used as maintenance medium for MDCK cell culture; synthetic nutrient medium supplemented by glutamine (0.584 g/L), 10% bovine serum, antibiotics (streptomycin 100 μg/cm3 and penicillin 100 U/cm3) was used as growth medium for and serum-free synthetic medium supplemented by antibiotics was used as maintenance medium for continuous Vero cell culture and primary trypsinized cell cultures.
Preparation of the material for inoculation into cell culture. The virus-containing suspension was filtered using a Millipore membrane filter (MCE) 20 microns (Merck Millipore, USA), centrifuged at 3,000 rpm for 15 minutes. Then, the supernatant was collected and antibiotics (streptomycin 100 μg/cm3 and penicillin 100 U/cm3) were added to the supernatant and the supplemented supernatant was left at a temperature of 2–8 °C for 1 hour and then used for inoculation in cell cultures.
The virus isolation was carried out in primary trypsinized baby dog kidney cell culture.
Inoculation of cell cultures. Three T25 culture flasks were used for each passage of the canine adenovirus serotype 2. Before virus inoculation onto the cell monolayer, the growth nutrient medium was decanted, the monolayer was washed three times with Hanks solution and then the virus-containing suspension was inoculated; the multiplicity of infection was 0.01 TCID50/cell. Cell culture flasks were placed in a thermostat at temperature of (37.0 ± 0.5) °C for 1 hour. Maintenance nutrient medium was added after 1 hour, cell culture flasks were placed in a thermostat at a temperature of (37.0 ± 0.5) °C and examined under an inverted microscope every 12 hours for characteristic morphological changes in cells: flasks showed more than 80% monolayer destruction were frozen at a temperature of minus (45 ± 5) °C until the next passage.
The following factors was also examined for their effect on the virus infectivity titre:
– cultivation time;
– cultivation temperature;
– age of cell culture monolayer;
– multiplicity of infection;
– preliminary holding time.
The virus was titrated according to the common procedure [23][24] in triplicate for each sample. For this purpose, Costar® (Corning, USA) 96-well flat-bottomed microplates were used. After adding the reaction components, the plates were cultivated at a temperature of (37.0 ± 0.5) °C and 5% CO2 concentration for 5 days (120 hours) and examined daily under an inverted microscope. The virus titre was assessed based on the number of wells with cytopathic effect. The titre was calculated using the Kärber method and expressed in lg TCID50/cm3.
Analysis of the results. The obtained examination results were processed using Microsoft Office Exсel programme.
RESULTS AND DISCUSSION
Pathological material selected for virus isolation was previously tested with PCR for CAV-2 viral genome detection; PCR-positive samples were used for further work. Primary trypsinized baby dog kidney cell culture was used for canine adenovirus serotype 2 isolation. Data on infectivity titres of the virus isolated at the first passage are presented in Table 1.
It was shown that all isolates found positive for CAV-2 genome by PCR, were infectious to different degree and were considered suitable for further work.
Five serial passages were carried out in primary trypsinized baby dog kidney cell culture to assess the virus reproducibility and stability. Test results are presented in Table 2.
Based on the test results, isolate No. 5 virus-containing material (later called “Yunity”) was selected for further cultivation, since it remained stable for five passages, the characteristic virus cytopathic effect (CPD) was observed at each passage level; the virus infectivity titre was in the range of (3.33 ± 0.29) up to (4.33 ± 0.29) lg TCID50/cm3. Virus-containing materials of isolates No. 1, 2, 3, 4 were not used for further testing since their titres were below 3.0 lg TCID50/cm3. At the next stages, third passage virus-containing material of isolate No. 5 with maximum titre for this isolate was used for testing the virus for its cultural properties.
At the first passage, CAV-2 caused CPE manifested 24 hours after the virus-containing suspension inoculation in the cell culture; 80% monolayer destruction was observed after 72 hours. At the second passage and subsequent passages, morphological changes in cells were detected after 24–48 hours, 80% monolayer destruction was registered after 72–120 hours.
Five serial passages were performed to test various cell cultures for their susceptibility to CAV-2. In susceptible cell cultures, the virus-induced CPE manifestations were similar and observed at the first passage. CAV-2 infectivity titre was determined by microtitration in MDCK cell culture. In case of cytopathic effect absence, the virus antigen in the culture fluid was detected using immunochromatographic test system. When the culture fluid was found negative during two passages, no further tests in the cell culture were performed. The virus infectivity titres in the selected cell cultures are given in Table 2 (for baby dog kidney cell culture) and Table 3 (for other cell cultures).
No CAV-2 accumulation was found in continuous Vero cell culture, primary trypsinized baby cat kidney and baby cat spleen cell cultures, no visible morphological changes were detected in these cells; no CAV-2 antigen was detected with immunochromatographic assay in culture fluid staring with the second passage. The virus titre was not determined during microtitration. The virus infectivity titre gradually decreased during the virus cultivation in the primary trypsinized baby dog spleen cell culture. Based on the obtained results the above cell cultures were considered non-suitable for the virus cultivation.
The virus infectivity in primary trypsinized baby dog kidney cell culture was (4.33 ± 0.29) lg TCID50/cm3 at the third passage; the virus titre in continuous MDCK cell culture was (4.25 ± 0.25) lg TCID50/cm3 at the fifth passage.
The following most susceptible cell cultures were identified based on the test results given in Tables 2 and 3: continuous MDCK cell cultures (NBL-2 line [22], the so-called parent line, and NBL-9 line), as well as the primary trypsinized baby dog kidney cell culture. However, use of a primary trypsinized culture for subsequent virus cultivation for specific vaccine and diagnosticum production is inexpedient since it is seasonal due to the use of tissue donors. Subcultivation of primary trypsinized cell cultures is impossible due to their low producibility.
Uneven cell distribution on the flask surface was noted during NBL-9 line MDCK monolayer formation that could potentially affect the virus infectivity accumulation and result in errors in CPE detection with microscopy (Fig. 1). The infectivity titre was slightly higher when the virus-containing material was cultivated in NBL-2 MDCK cell culture. Thus, continuous MDCK cells (NBL-2 line) was selected for further tests aimed at determination of optimal virus cultivation conditions (Fig. 2).
Intact MDCK cell culture (NBL-2 line) is shown in Figure 3. Figures 4 and 5 show the virus-induced CPE manifestations after 96 and 120 hours (magnification 200× and 400×): individual rounded refractive cells gradually detaching from the glass are clearly visible. At the beginning, cells showed focal morphological changes, then the monolayer destroyed and the cell detached from the culture flask surface. As the virus multiplied, the number of cells undergoing degeneration increased and voids formed in the monolayer. The affected cells concentrated along the edges of the remained monolayer areas, forming large conglomerates resembling grape bunches.
Cultivation time required for CAV-2 accumulation at maximum titres was to be determined for further testing. Test results are presented in Table 4.
It was found that the virus accumulated to maximum levels (4.08 ± 0.29 lg TCID50/cm3) on day 5 of cultivation. The virus reproduction ranged from (1.25 ± 0.25) to (3.08 ± 0.29) lg TCID50/cm3 when the virus was cultivated for 48, 72 and 96 hours. The virus infectivity titre decreased to (3.42 ± 0.14) and (3.08 ± 0.14) lg TCID50/cm3 when the virus was cultivated for 144 and 168 hours, respectively. The decrease in the virus infectivity was accounted for slowdown in the metabolic processes in cells linked with the external environment during long-term cultivation.
Also, temperature (39.0 ± 0.5) °C was preliminary examined for its effect on MDCK cell culture viability. At this temperature, morphological changes were observed in the cell culture after 12 hours. After 24 hours, the monolayer detached from the surface. Therefore, this temperature was not used for the virus cultivation.
Results presented in Table 5, show that CAV-2 activity was lower and the maximum titre was (2.83 ± 0.29) lg TCID50/cm3 after 144 hours of cultivation at a temperature of (35.0 ± 0.5) °C. When the virus was cultivated at temperature (37.0 ± 0.5) °C in MDCK cell culture (NBL-2 line), the virus activity was maximum (4.33 ± 0.14 lg TCID50/cm3) after 120 hours of cultivation. Then, CAV-2 infectivity titre gradually decreased.
At the next stage, the optimal age of the cell monolayer for virus inoculation was determined. For this purpose, monolayers formed 24, 48, 72, 96 hours after the MDCK cells were placed into culture flask and the cell culture that was placed into culture flask immediately before inoculation of the virus-containing material were used. The results were recorded after 120 hours (5 days) of incubation in a thermostat at a temperature of (37.0 ± 0.5) °C.
Based on the obtained data (Table 6), the optimal time for the cell culture monolayer formation to be used for the virus inoculation was 48 hours, the virus titre was (4.08 ± 0.38) lg TCID50/cm3. When CAV-2 was inoculated into the cell suspension before monolayer formation, the virus titre was (2.42 ± 0.14) lg TCID50/cm3, the monolayer formed more slowly than in the intact cell culture, and the trend for formation of cell clusters was more evident. Cell metabolism and virus reproduction appeared to decrease during cultivation for 72 and 96 hours. The infectivity titre was (3.83 ± 0.14) and (3.08 ± 0.29) lg TCID50/cm3, respectively.
The following infection doses were used to test the effect of the multiplicity of infection on CAV-2 accumulation level in MDCK cell culture (NBL-2 line): 0.1; 0.01; 0.001; 0.0001 TCID50/cell. Incubation was stopped when 80% of the cell monolayer area destroyed and the cells detached from the surface. The obtained results are shown in Table 7.
When the infection dose was 0.1 TCID50/cell, CPE was observed as early as 48 hours after the virus suspension inoculation into the cell culture. However, CAV-2 titre was (3.08 ± 0.38) lg TCID50/cm3 and was lower than that one (4.33 ± 0.29 lg TCID50/cm3) when the infection dose of 0.01 TCID50/cell was used that was accounted for the rapid monolayer destruction and, as a result, the lack of the possibility for the virus to accumulate to its maximum concentration. At a multiplicity of infection of 0.001 and 0.0001 TCID50/cell, the virus infectivity titre was low and was (2.17 ± 0.14) and (1.75 ± 0.25) lg TCID50/cm3, respectively; CPE was observed in cell culture after 96 hours.
Duration of preliminary holding (adsorption) of the virus with the MDCK cell culture monolayer is also a factor of interest in view of its effect on the virus accumulation.
CAV-2 infectivity titre was found to be (2.16 ± 0.14) lg TCID50/cm3 when the cultivation was carried out without adsorption. Preliminary holding of the virus with the monolayer enhanced CAV-2 reproduction manifested by an increase in its infectivity titre. Preliminary holding for 60 minutes was the optimal time for maximum virus accumulation: the infectivity titre was (4.33 ± 0.29) lg TCID50/cm3. When preliminary adsorption time was 30 and 90 minutes, the virus infectivity titre was (4.08 ± 0.38) and (4.08 ± 0.14) lg TCID50/cm3, respectively.
Table 1
CAV-2 infectivity in primary trypsinized baby dog kidney cell culture when the virus was isolated at the first passage
|
Isolate No. |
Infectivity titre, lg TCID50/cm3 |
|
1 |
1.83 ± 0.14 |
|
2 |
1.67 ± 0.29 |
|
3 |
1.92 ± 0.38 |
|
4 |
2.42 ± 0.29 |
|
5 |
3.33 ± 0.29 |
Table 2
Serial CAV-2 cultivation in primary trypsinized baby dog kidney cell culture
|
Virus passage No. |
Infectivity titre, lg TCID50/cm3 |
||||
|
Isolate No. 1 |
Isolate No. 2 |
Isolate No. 3 |
Isolate No. 4 |
Isolate No. 5 |
|
|
1 |
1.83 ± 0.14 |
1.67 ± 0.29 |
1.92 ± 0.38 |
2.42 ± 0.29 |
3.33 ± 0.29 |
|
2 |
0.83 ± 0.58 |
1.67 ± 0.29 |
2.25 ± 0.25 |
2.50 ± 0.43 |
4.17 ± 0.14 |
|
3 |
– |
1.17 ± 0.14 |
2.50 ± 0.25 |
2.17 ± 0.14 |
4.33 ± 0.29 |
|
4 |
– |
– |
2.58 ± 0.14 |
1.75 ± 0.25 |
4.08 ± 0.14 |
|
5 |
– |
– |
2.33 ± 0.29 |
– |
3.92 ± 0.14 |
|
“–” – not tested. |
|||||
Table 3
Susceptibility of different continuous and primary trypsinized cell cultures to CAV-2 Yunity isolate
|
Cell culture |
Infectivity titre, lg TCID50/cm3 |
||||
|
1st passage |
2nd passage |
3rd passage |
4th passage |
5th passage |
|
|
Continuous cell cultures |
|||||
|
MDCK NBL-2 |
4.33 ± 0.29 |
4.00 ± 0.25 |
4.08 ± 0.14 |
4.17 ± 0.14 |
4.25 ± 0.25 |
|
MDCK NBL-9 |
4.00 ± 0.25 |
3.92 ± 0.38 |
3.83 ± 0.29 |
4.00 ± 0.00 |
3.83 ± 0.14 |
|
Vero |
< 1.0* |
< 1.0 |
– |
– |
– |
|
Primary trypsinized cell cultures |
|||||
|
BDS |
2.42 ± 0.14 |
2.17 ± 0.29 |
1.50 ± 0.25 |
1.17 ± 0.29 |
0.92 ± 0.14 |
|
BCK |
< 1.0 |
< 1.0 |
– |
– |
– |
|
BCS |
< 1.0 |
< 1.0 |
– |
– |
– |
|
* microtitration results; “–” – not tested; BDS– baby dog spleen; BCK – baby cat kidney; BCS – baby cat spleen. |
|||||
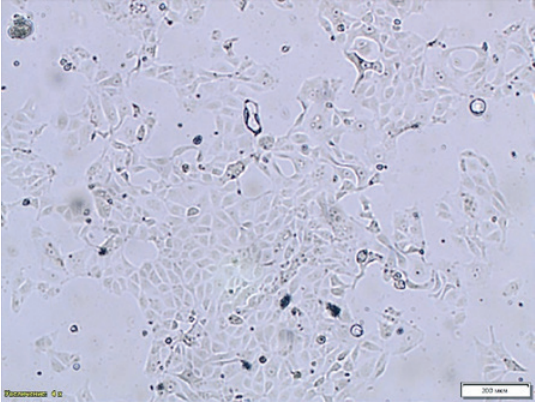
Fig. 1. Monolayer formation – age: 24 hours, MDCK cell culture NBL-9 line (200× magnification)
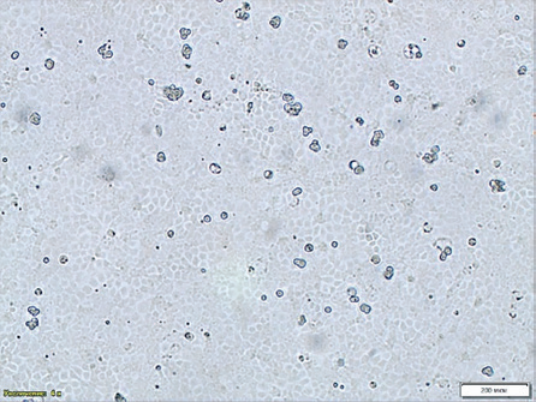
Fig. 2. Monolayer formation – age: 24 hours, MDCK cell culture NBL-2 line (200× magnification)
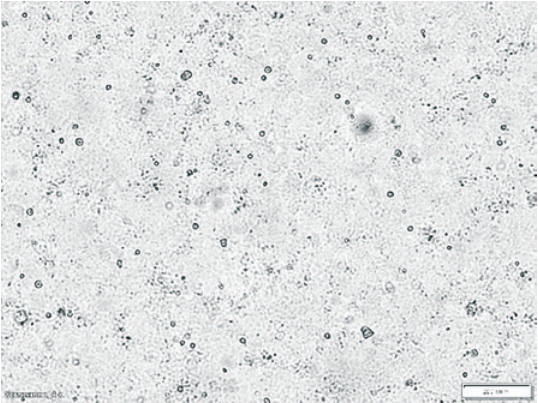
Fig. 3. Intact continuous MDCK cell culture NBL-2 line (200× magnification)
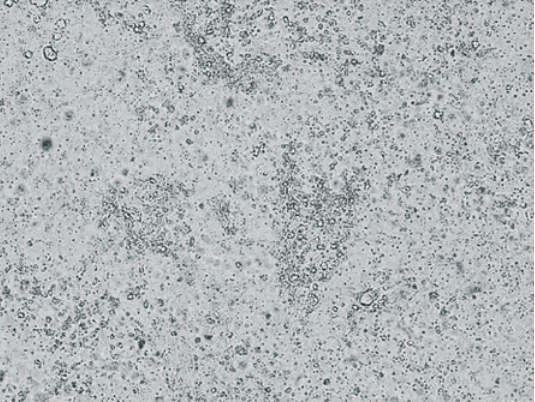
Fig. 4. CAV-2 CPE manifestation 96 hours after infection of continuous MDCK cell culture NBL-2 line (200× magnification)
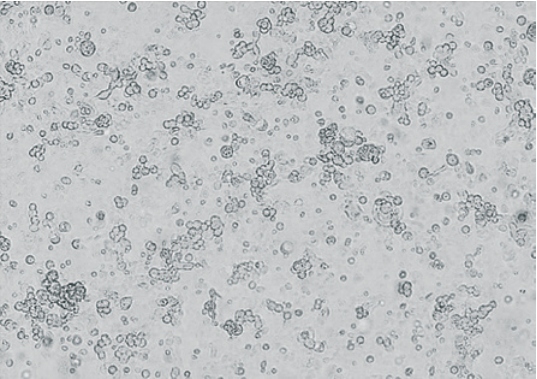
Fig. 5. CAV-2 CPE manifestation 120 hours after infection of continuous MDCK cell culture NBL-2 line (400× magnification)
Table 4
CAV-2 accumulation depending on the time of cultivation in continuous MDCK NBL-2 line cell culture
|
Cultivation time, hours |
Infectivity titre, lg TCID50/cm3 |
|
48 |
1.25 ± 0.25 |
|
72 |
2.92 ± 0.38 |
|
96 |
3.08 ± 0.29 |
|
120 |
4.08 ± 0.29 |
|
144 |
3.42 ± 0.14 |
|
168 |
3.08 ± 0.14 |
Table 5
CAV-2 infectivity titre depending on temperature of cultivation in continuous MDCK NBL-2 line cell culture
|
Cultivation time |
Infectivity titre, lg TCID50/cm3 |
|
|
(35.0 ± 0.5) °C |
(37.0 ± 0.5) °C |
|
|
1 day (24 hours) |
0 |
0 |
|
2 days (48 hours) |
0.92 ± 0.14 |
1.17 ± 0.14 |
|
3 days (72 hours) |
2.67 ± 0.14 |
3.33 ± 0.14 |
|
4 days (96 hours) |
2.75 ± 0.25 |
3.58 ± 0.14 |
|
5 days (120 hours) |
2.83 ± 0.14 |
4.33 ± 0.29 |
|
6 days (144 hours) |
2.83 ± 0.29 |
3.58 ± 0.29 |
|
7 days (168 hours) |
1.75 ± 0.00 |
2.17 ± 0.14 |
Table 6
Correlation of MDCK NBL-2 line cell culture monolayer age with CAV-2 infectivity titre
|
Monolayer formation time, hours |
Infectivity titre, lg TCID50/cm3 |
|
0 |
2.42 ± 0.14 |
|
24 |
3.17 ± 0.14 |
|
48 |
4.08 ± 0.38 |
|
72 |
3.83 ± 0.14 |
|
96 |
3.08 ± 0.29 |
Table 7
CAV-2 infectivity titre depending on infectious dose in MDCK NBL-2 line cell culture
|
MOI, TCID50/cell |
Cultivation time, hours |
Infectivity titre, lg TCID50/cm3 |
|
0.1 |
48 |
3.08 ± 0.38 |
|
0.01 |
72 |
4.33 ± 0.29 |
|
0.001 |
96 |
2.17 ± 0.14 |
|
0.0001 |
96 |
1.75 ± 0.25 |
|
MOI – multiplicity of infection. |
||
CONCLUSION
Canine adenovirus serotype 2 was isolated as a result of the study, the isolated virus remained stable for five passages and had high infectivity titre.
CAV-2 isolate was tested for its cultivation parameters in continuous and primary trypsinized cell cultures. MDCK cell culture (NBL-2 line) was selected as the most susceptible culture enabling yielding of the virus-containing material at high titre (4.33 ± 0.29 lg TCID50/cm3). The following conditions facilitating CAV-2 accumulation to maximum titres were found optimal: use of the cell culture monolayer formed within 48 hours for the virus inoculation; a multiplicity of infection of 0.01 TCID50/cell; preliminary holding time – 60 minutes; cultivation at a temperature of (37.0 ± 0.5) °C for 120 hours.
The obtained results can be used for development of diagnostic test systems and vaccines for prevention of CAV-2 infection in dogs.
References
1. Savina N. N., Ekimov A. A., Trukhin V. P., Evtushenko A. E., Zhirenkina E. N., Sinegubova E. O., Slita A. V. Evaluation of avian adenovirus inactivation methods used in the production of influenza vaccines. Extreme Medicine. 2021; (3): 78–83. https://doi.org/10.47183/mes.2021.032
2. Chander V., Sharma G. K., Bhatt M., Nandi S., Mahajan S., Singh M., et al. Isolation and genetic characterization of canine adenovirus type 2 from a domestic dog showing neurological symptoms. Brazilian Journal of Microbiology. 2021; 52 (4): 2521–2528. https://doi.org/10.1007%2 Fs42770-021-00540-0
3. Ford R. B., Vaden S. L. Canine infectious tracheobronchitis. In: Infectious Diseases of the Dog and Cat. Ed. by C. E. Greene. 2nd ed. Philadelphia: W. B. Saunders Company; 1998; 33–38.
4. ICTV. Taxon Details. https://ictv.global/taxonomy/taxondetails?taxnode_id=202302418&taxon_name=Mastadenovirus%20canidae
5. Ditchfield J., Macpherson L. W., Zbitnew A. Association of canine adenovirus (Toronto A 26/61) with an outbreak of laryngotracheitis (“Kennel Cough”): A preliminary report. Canadian Veterinary Journal. 1962; 3 (8): 238–247. https://pubmed.ncbi.nlm.nih.gov/17421510
6. Barrett T., Beard P., Clegg J. C. S., Gould E. A., Hull R., Inglis S. C. et al. Virology: A Practical Approach. Ed. by B. W. J. Mahy. Oxford, Washington: IRL Press Ltd.; 1985. 264 p.
7. Diagnosis and prevention of infectious dog and cat diseases: guidelines for veterinary practitioners. Ed. by T. I. Aliper. Moscow: ZooVetKniga; 2017. 304 p. (in Russ.)
8. Macartney L., Cavanagh H. M. A., Spibey N. Isolation of canine adenovirus-2 from the faeces of dogs with enteric disease and its unambiguous typing by restriction endonuclease mapping. Research in Veterinary Science. 1988; 44 (1): 9–14. https://doi.org/10.1016/0034-5288(88)90004-5
9. Ramidi A., Ganji V. K., Buddala B., Yella N. R., Manthani G. P., Putty K. E3 gene-based genetic characterization of canine adenovirus-2 isolated from cases of canine gastroenteritis in India revealed a novel group of the virus. Intervirology. 2020; 62 (5–6): 216–221. https://doi.org/10.1159/000507329
10. Buonavoglia C., Martella V. Canine respiratory viruses. Veterinary Research. 2007; 38 (2): 355–373. https://doi.org/10.1051/vetres:2006058
11. Zhu Y., Xu J., Lian S., Zhang R., Hou J., Wang M., Yan X. Difference analysis between canine adenovirus types 1 and 2. Frontiers in Cellular and Infection Microbiology. 2022; 12:854876. https://doi.org/10.3389/fcimb.2022.854876
12. Day M. J., Carey S., Clercx C., Kohn B., MarsilIo F., Thiry E., et al. Aetiology of canine infectious respiratory disease complex and prevalence of its pathogens in Europe. Journal of Comparative Pathology. 2020; 176: 86–108. https://doi.org/10.1016/j.jcpa.2020.02.005
13. Dowgier G., Lahoreau J., Lanave G., Losurdo M., Varello K., Lucente M. S., et al. Sequential circulation of canine adenoviruses 1 and 2 in captive wild carnivores, France. Veterinary Microbiology. 2018; 221: 67–73. https://doi.org/10.1016/j.vetmic.2018.05.025
14. Timurkan M. O., Aydin H., Alkan F. Detection and molecular characterization of canine adenovirus type 2 (CAV-2) in dogs with respiratory tract symptoms in shelters in Turkey. Veterinarski Arhiv. 2018; 88 (4): 467–479. https://doi.org/10.24099/vet.arhiv.0052
15. Koptopoulos G., Cornwell H. J. C. Canine adenoviruses: a review. Veterinary Bulletin. 1981; 51 (3): 135–142. https://www.cabidigitallibrary.org/doi/pdf/10.5555/19812282132
16. Vieson M. D., Piñeyro P., LeRoith T. A review of the pathology and treatment of canine respiratory infections. Veterinary Medicine: Research and Reports. 2012; 3: 25–39. https://doi.org/10.2147/vmrr.s25021
17. O’Neill D., Jackson C., Guy J., Church D., McGreevy P., Thomson P., Brodbelt D. Epidemiology of canine upper respiratory disorders in brachycephalic and non-brachycephalic breeds attending veterinary practices in England. BSAVA Congress Proceedings (9–12 April, 2015). Birmingham: British Small Animal Veterinary Association; 2015; 481–482. https://doi.org/10.22233/9781910443521.65.4
18. Balboni A., Mollace C., Giunti M., Dondi F., Prosperi S., Battilani M. Investigation of the presence of canine adenovirus (CAdV) in owned dogs in Northern Italy. Research in Veterinary Science. 2014; 97 (3): 631–636. https://doi.org/10.1016/j.rvsc.2014.10.010
19. Priestnall S. L., Mitchell J. A., Walker C. A., Erles K., Brownlie J. New and emerging pathogens in canine infectious respiratory disease. Veterinary Pathology. 2014; 51 (2): 492–504. https://doi.org/10.1177/0300985813511130
20. Gaush C. R., Hard W. L., Smith T. F. Characterization of an established line of canine kidney cells (MDCK). Proceedings of the Society for Experimental Biology and Medicine. 1966; 122 (3): 931–935. https://doi.org/10.3181/00379727-122-31293
21. American Type Culture Collection. https://www.atcc.org
22. Valentich J. D. Morphological similarities between the dog kidney cell line MDCK and the mammalian cortical collecting tubule. Annals of the New York Academy of Sciences. 1981; 372 (1): 384–405. https://doi.org/10.1111/j.1749-6632.1981.tb15490.x
23. Klimova A. A., Komarova A. A., Kiselev A. M., Galkina T. S. Methodical guidelines for canine adenovirus microtitration approved by the Federal Centre for Animal Health 14.06.2022 No. 35–22. Vladimir; 2022. 11 p. (in Russ.)
24. Glinskaya E. V., Tuchina E. S., Petrov S. V. Virology. Methodical materials: study guide. Saratov; 2013. 84 p. (in Russ.)
About the Author
A. A. KlimovaRussian Federation
Anastasia A. Klimova, Veterinarian, Laboratory for Pets Disease Prevention
Yur’evets, Vladimir 600901, Russia
Review
For citations:
Klimova A.A. Canine adenovirus serotype 2 isolation and determination of its cultivation parameters. Veterinary Science Today. 2024;13(4):352-359. https://doi.org/10.29326/2304-196X-2024-13-4-352-359



































