Scroll to:
Study of possible intrauterine infection of goat fetus with caprine arthritis-encephalitis virus
https://doi.org/10.29326/2304-196X-2024-13-4-338-343
Abstract
Caprine arthritis-encephalitis is a serious challenge for the modern goat breeding both in Russia and abroad. The disease is quite widespread in our country, causing serious economic damage to the backyard and family-operated farm owners. The etiologic agent of caprine arthritis-encephalitis (CAE) is a virus of the Retroviridae family, which is part of the group of small ruminant lentiviruses subdivided into five genetic groups. A number of reasons prevent timely disease diagnosis, for example, lack of a legal framework regulating preventive measures, prolonged asymptomatic virus-carrier state, and absence of pathognomonic symptoms. There are two routes of the CAE virus spread: vertical (lactogenic), when colostrum or milk from a seropositive goat serves as a transmission factor; and horizontal – from a diseased animal to a healthy one in case of crowded housing, feeding from common drinkers and feeders, as well as during mating. The published data on the possibility of the intrauterine virus transmission from mother to fetus are diametrically different. The purpose of this study was to explore the possibility of intrauterine infection with the CAE virus. Pregnant goats with ELISAand PCR-confirmed CAE diagnosis were used in the study. Pathological material was collected from newborn goats obtained by sterile kidding, and the samples were PCR tested for the CAE causative agent. None of the tested samples demonstrated CAE that gives evidence of absence of the fact of intrauterine virus transmission from the diseased mother to the fetus. Results of long-term monitoring of the goat population kept on two backyards located in the Southern Federal District and Novosibirsk Oblast comprise an indirect evidence of that. The owners of these backyards, where 100% of the goat population were infected with CAE virus, decided to carry out a complex of the disease control measures. Sterile kidding followed by kids’feeding with colostrum and milk pasteurized at 60 °C for 30 minutes with the subsequent use of a whole milk substitute allowed to obtain a CAE-free herd in two years avoiding any significant economic losses and purchase of healthy animals from other farms.
Keywords
For citations:
Koptev V.Yu., Shkil N.A., Balybina N.Yu., Belenkova T.N. Study of possible intrauterine infection of goat fetus with caprine arthritis-encephalitis virus. Veterinary Science Today. 2024;13(4):338-343. https://doi.org/10.29326/2304-196X-2024-13-4-338-343
INTRODUCTION
Caprine arthritis-encephalitis (CAE) is one of the burning issues of the modern goat breeding in Russia and abroad. According to the published data, the carriers of the disease causative agent are more than 45–60% of the entire population of the goats kept on the backyards and family-operated farms located in all federal districts of the Russian Federation [1].
The CAE etiological factor is a virus of the Retroviridae family, which is part of the group of small ruminant lentiviruses (SRLVs), divided into five genetic groups [2]. The disease belongs to the group of lentiviral infections and it is characterized by prolonged asymptomatic virus carrying with the subsequent development of a complex of symptoms involving lesions of the musculoskeletal system (arthritis), respiratory system and mammary gland tissue. Young animals of 2–3 months of age demonstrate disorders of the central nervous system, manifested by loss of orientation, tilting of the head and incoordination [3-6].
There are vertical and horizontal routes of the infectious agent transmission. The animals are infected lactogenically when newborn kids are fed with the colostrum or milk from CAE-virus carrier goats [7-9], as well as by airborne route in case of crowded housing, and less often sexually [10][11][12].
The published opinions regarding the intrauterine route of the virus transmission differ. Volkova I. Yu. [13] indicates that this route of infection is possible. However, in a number of publications the authors say that presence of syndesmochorial type of placenta in goats prevents the virus transmission from mother to fetus [3][8][14][15]. Nevertheless, some foreign publications provide data on the virus detection during the examination of newborn kids using polymerase chain reaction (PCR) [16][17].
Despite this, the World Organisation for Animal Health’s (WOAH) Terrestrial Animal Health Code does not provide information on a possible intrauterine route of CAE virus transmission [18].
Lack of specific therapy and disease prevention tools hinders the implementation of highly effective mass measures to prevent the spread of this pathology in the goat population. The main method of the disease prevention and control on the farms involves continuous monitoring of the entire population, and in case of detection of seropositive animals – taking a set of measures to replace the diseased herd with the healthy one. One of such measures involves introduction of sterile kidding, i.e. a set of measures to prevent direct contact of a seropositive goat with a newborn kid, followed by feeding it with disinfected colostrum and milk.
In view of the diametrically opposite information in the published literature, as well as taking into account the fact that currently CAE is included in the “List of contagious animal diseases, including highly dangerous ones, for which quarantine can be imposed”1, the purpose of the study was formulated as follows: to study the possibility of intrauterine infection of newborn kids with CAE virus.
MATERIALS AND METHODS
The study was carried out in 2023–2024 in the Laboratory of Diseases of Young Animals of the Experimental Veterinary Medicine Institute for Siberia and Far East of the Siberian Federal Scientific Centre of Agro-BioTechnologies of the Russian Academy of Sciences and on a family-operated farm located in one of the regions of the Ural Federal District. Eighteen pregnant CAE-seropositive Saanen goats were used in the study. The animals were diagnosed by testing blood samples for the proviral DNA using PCR, as well as by double testing of the sera by the enzyme-linked immunosorbent assay (ELISA) for the antibodies to CAE virus a week before insemination and on day 60 of pregnancy.
The blood samples from female goats were collected in Bodywin vacuum tubes (China) with coagulation activator and ethylenediaminetetraacetic acid.
Presence of CAE virus antibodies in the sera was determined using the ID Screen® MVV/CAEV Indirect Screening test kits for indirect ELISA (IDVet, France). The results were recorded on a semi-automatic microplate ELISA reader TECAN Infinite F50 (Austria).
Euthanasia of newborn animals was carried out in accordance with the requirements of the European Convention for the Protection of Pet Animals (Chapter 2, Article 11)2. Stunning method was used for euthanasia [19].
Autopsy of the newborn kids was carried out using the generally accepted method of G. V. Shor [20]. Blood and internal organ samples were aseptically collected in sterile test tubes using sterile disposable sample collecting probes (VetGenomics, Russia), as well as biological sample collection and storage cards “DNA Archive” (Russia).
For primary isolation of nucleic acids from the biological material, “RealBest extraction 100” kit (Vector-Best, Russia) was used.
Caprine arthritis-encephalitis virus was detected in the biological samples using recording amplifier manufactured by Bio-Rad Laboratories, Inc. (USA) and a “Kit of reagents for detection of proviral DNA of caprine arthritis encephalitis virus (CAE) using real-time polymerase chain reaction” (Vector Best JSC, Russia). Fifty amplification cycles were performed. Samples with Ct < 40 were considered positive.
RESULTS AND DISCUSSION
Before the study, all the goats (n = 50) housed on one of the family-operated farms were tested for CAE virus antibodies using ELISA before the insemination. Polymerase chain reaction for proviral DNA was used as a confirmation test. Goats with a seropositivity coefficient of Cs ≥ 100 (according to ELISA results) and positive PCR test results (n = 29) were selected for further study. Then, on day 60 of the pregnancy, a second ELISA was performed to confirm the CAE diagnosis in the goats.
During the kidding, the newborn kids were aseptically taken and removed, completely excluding their postpartum contact with the mother. Only males were used in the experiment, since they do not have any commercial value. A total of 18 goats were selected for the study (Fig.).
After euthanasia, an autopsy and biological material collection were aseptically performed. The following samples were collected: blood samples, heart tissue, liver, lungs. The biological materials were stored and transported at 4 °C.
Further work with the biological samples was carried out in the PCR laboratory of the Experimental Veterinary Medicine Institute for Siberia and Far East of the Siberian Federal Scientific Centre of Agro-BioTechnologies of the Russian Academy of Sciences
The test results are demonstrated in Table 1.
The CAE virus (CAEV) was not detected in any of the biological samples tested using real-time PCR. This fact confirmed that intrauterine infection of newborn kids by the virus-carrying mothers is impossible.
The data obtained during the experiment differ from the information cited in a number of foreign publications. Thus, J. Furtado Araújo et al. [17] indicate that when testing 73 newborn kids by PCR, the CAEV was detected in 46.57% of the samples. One of the explanations for the discrepancy between our results and the cited published data can be a comment from the article by O. L. Kolbasova et al. [2] that the quality of PCR directly depends on the primers, which should match the genetic variant of the virus circulating in the animals housed
on the farm.
During the experiment, all goats were tested using a combination of ELISA and PCR diagnostic tests, and the animals that demonstrated double positive results were selected for the experiment. Therefore, the complex of primers included in the PCR system used in the experiment matched the genetic variant of the virus circulating on the farm.
The data confirm the relevance of using the sterile kidding (complete exclusion of any contact of the mother and newborn kid immediately after parturition) as one of the main approaches to the prevention of CAE spread inside the farm and to the formation of the disease-free herd.
The following examples confirm the effectiveness of this method and, as a result, the absence of an intrauterine route of CAEV transmission.
Example 1. The experiment was conducted on the backyard located in the Southern Federal District. The number of goats at the beginning of the experiment was 18 (Table 2).
The presented data demonstrate that in 2022, during the initial examination of the population consisting of 18 sexually mature animals, the antibodies to the CAE virus were detected in the sera of 11 goats. The backyard owners decided to improve the health of the herd, therefore, they put into practice mandatory sterile kidding for all pregnant goats and feeding newborn kids with colostrum pasteurized at 60 °C for 30 minutes, with further use of the whole milk substitute. Four goats with obvious CAE clinical signs were isolated from the herd, the rest were mated by the available seropositive male goats.
In 2023, when examining the repair population formed of the kids born by sterile kidding (13 kids), all animals demonstrated negative results. The only seropositive animal turned out to be a female goat, which the owners wanted to use for mating again, but as soon as they received data that all the goats were seronegative, it was decided to send it to slaughter.
In 2024, upon repeated double ELISA testing of the newly formed goat population, all animals demonstrated negative results for CAE virus antibodies.
Example 2. The experiment was carried out on the backyard located in the Novosibirsk Oblast. At the beginning of the monitoring, the herd consisted of 24 Nubian dairy goats (Table 3).
During the initial ELISA testing of the animals in 2020, it was found that the entire goat herd was infected with the CAE virus.
The owners of the farm decided to carry out a set of measures to improve the health of the herd. To do this, all subsequent kiddings were carried out using sterile kidding technology, and the resulting kids were fed with the colostrum pasteurized at 60 °C for 30 min and with milk derived from mother goats.
Testing of the young animals obtained using this technology in 2021 demonstrated that 12 out of 20 animals were the CAE virus carriers. When interviewing the owners, it was found that after the end of the period of feeding with milk, the animals were placed in the common herd at the age of 3 months, which resulted in their infection with the CAE virus from the diseased mother goats.
Given this fact, the backyard owners housed all kids derived from seropositive goats in 2022 in a separate room, completely excluding any contact with infected animals and items of their care. As a result, during the ELISA testing of the newly formed herd (6–7 months of age), all animals (23 goats) demonstrated no CAEV antibodies in their sera. The owners decided to cull all seropositive animals, disinfect the premises and form a new herd of seronegative young animals.
The repeated double tests in 2023 confirmed the absence of CAE virus circulation in the entire goat herd on this backyard. In 2024, the owners ELISA tested all animals again at a six-month interval and the results were negative.
The data obtained during the experiment, as well as the results of the monitoring tests given in the examples, confirm the effectiveness of sterile kidding as the main method of preventing the CAE spread on the goat farms. Strict compliance with all procedures makes it possible to obtain a healthy offspring with high breeding and economic value from virus-carrying animals.
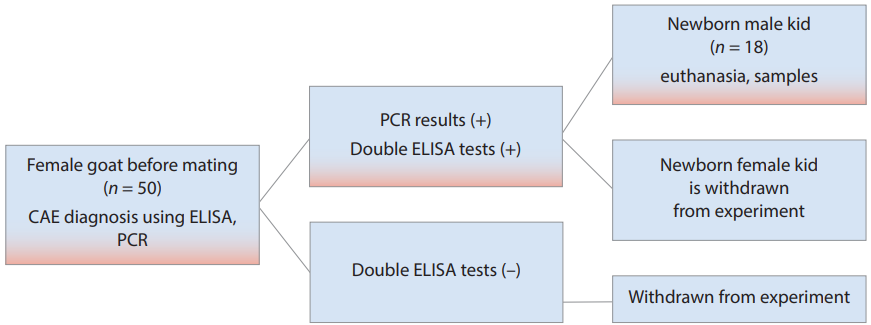
Fig. Scheme of selecting newborn animals for the experiment
Table 1
Results of biological material tests for CAE virus
|
Type of biological material |
Amount |
CAEV test results |
|
|
positive, % |
negative, % |
||
|
Blood samples |
18 |
– |
100 |
|
Internal organ samples |
54 |
– |
100 |
Table 2
Changes in the number of seropositive animals in the goat population on backyard No. 1
|
Year |
Number of tested animals |
CAEV antibody test results, animals |
|
|
seropositive |
seronegative |
||
|
2022 |
18 |
11 |
7 |
|
2023 |
14 |
1 |
13 |
|
2024 |
13 |
– |
13 |
Table 3
Changes in the number of seropositive animals in the goat population on backyard No. 2
|
Year |
Number of tested animals |
CAEV antibody test results, animals |
|
|
seropositive |
seronegative |
||
|
2020 |
24 |
24 |
– |
|
2021 |
20 |
12 |
8 |
|
2022 |
23 |
– |
22 |
|
2023 |
23 |
– |
23 |
|
2024 |
23 |
– |
23 |
CONCLUSION
Currently, the scientific literature provides contradictory data on the possibility of intrauterine infection of goats with the caprine arthritis-encephalitis virus. A number of foreign authors cite the research results indicating CAE agent isolation from newborn kids derived from seropositive animals. At the same time, there is no data in the domestic literature, as well as in the WOAH Terrestrial Animal Health Code on possible intrauterine route of CAE infection.
The results of our tests of biological materials collected from newborn kids derived from CAE seropositive goats indicate that the syndesmochorial type of placenta characteristic of small ruminants is a natural barrier to small ruminant lentiviruses, which excludes intrauterine transmission of the caprine arthritis-encephalitis causative agent. Given this fact, as well as the fact that currently there are no means of specific prevention and therapy of caprine arthritis-encephalitis, the only way to prevent the disease are technological methods, in particular sterile kidding, which excludes the virus transmission from the mother to the newborn kid.
This fact has been confirmed by long-term monitoring of the number of goats kept on two farms located in the Southern Federal District and in the Novosibirsk Oblast for the presence of CAE seropositive animals in the herd. In both cases, the owners’ transition to the use of sterile kidding, which excludes any contact of the newborn kids with the seropositive mothers, within two years allowed for complete removal of the infected animals from the herd and replacement of all population with seronegative animals thus avoiding economic losses and purchase of healthy animals from other farms.
The presented data confirm that the use of sterile kidding followed by feeding the offspring with colostrum pasteurized at 60 °C for 30 minutes and milk is currently the only way to prevent the CAE spread inside the goat herd kept on the same farm.
Nevertheless, given the small sample of animals used in the experiment, as well as the fact that published opinions on the possibility of intrauterine infection differ diametrically, further research is needed, which should cover more animals.
1. The list of contagious animal diseases, including highly dangerous ones, for which quarantine can be imposed: approved by Order of the Ministry of Agriculture of the Russian Federation No. 476 of 19 December 2011 (as amended on 25 September 2020). https://docs.cntd.ru/document/902324591
2. European Convention for the Protection of Pet Animals. Strasbourg, 13.XI.1987. https://rm.coe.int/168007a67d
References
1. Koptev V. Yu., Shkil N. А., Balybina N. Yu., Penkova I. N. Clinical signs of caprine arthritis-encephalitis and disease-related pathomorphological changes. Veterinary Science Today. 2023; 12 (2): 126–132. https://doi.org/10.29326/2304-196X-2023-12-2-126-132
2. Kolbasova O. L., Bespalova T. Yu., Korogodina E. V., Krasnova E. A. Сaprine arthritis-encephalitis: early diagnostics topical issues. Veterinaria Kubani. 2023; (2): 23–25. https://doi.org/10.33861/2071-8020-2023-2-2325 (in Russ.)
3. Minguijón E., Reina R., Pérez M., Polledo L., Villoria M., Ramírez H., et al. Small ruminant lentivirus infections and diseases. Veterinary Microbiology. 2015; 181 (1–2): 75–89. https://doi.org/10.1016/j.vetmic.2015.08.007
4. Kudryashov A. A., Balabanova V. I., Babina S. Yu. Pathomorphological findings in lungs and brain at caprine arthritis-encephalitis. Actual Questions of Veterinary Biology. 2014; (3): 54–58. https://elibrary.ru/spcqyj (in Russ.)
5. Czopowicz M., Szaluś-Jordanow O., Moroz A., Mickiewicz M., Witkowski L., Markowska-Daniel I., et al. Use of two commercial caprine arthritisencephalitis immunoenzymatic assays for screening of arthritic goats. Journal of Veterinary Diagnostic Investigation. 2017; 30 (1): 36–41. https://doi.org/10.1177/1040638717729397
6. Benavides J., González L., Dagleish M., Pérez V. Diagnostic pathology in microbial diseases of sheep or goats. Veterinary Microbiology. 2015; 181 (1–2): 15–26. https://doi.org/10.1016/j.vetmic.2015.07.012
7. Pisoni G., Bertoni G., Manarolla G., Vogt H.-R., Scaccabarozzi L., Locatelli C., Moroni P. Genetic analysis of small ruminant lentiviruses following lactogenic transmission. Virology. 2010; 407 (1): 91–99. https://doi.org/10.1016/j.virol.2010.08.004
8. Peterhans E., Greenland T., Badiola J., Harkiss G., Bertoni G., Amorena B., et al. Routes of transmission and consequences of small ruminant lentiviruses (SRLVs) infection and eradication schemes. Veterinary Research. 2004; 35 (3): 257–274. https://doi.org/10.1051/vetres:2004014
9. Blacklaws B. A., Berriatua E., Torsteinsdottir S., Watt N. J., de Andres D., Klein D., Harkiss G. D. Transmission of small ruminant lentiviruses. Veterinary Microbiology. 2004; 101 (3): 199–208. https://doi.org/10.1016/j.vetmic.2004.04.006
10. Illius A. W., Lievaart-Peterson K., McNeilly T. N., Savill N. J. Epidemiology and control of maedi-visna virus: Curing the flock. PLoS ONE. 2020; 15 (9):e0238781. https://doi.org/10.1371/journal.pone.0238781
11. Broughton-Neiswanger L. E., White S. N., Knowles D. P., Mousel M. R., Lewis G. S., Herndon D. R., Herrmann-Hoesing L. M. Non-maternal transmission is the major mode of ovine lentivirus transmission in an ewe flock: A molecular epidemiology study. Infection, Genetics and Evolution. 2010; 10 (7): 998–1007. ttps://doi.org/10.1016/j.meegid.2010.06.007
12. Kalogianni A. I., Stavropoulos I., Chaintoutis S. C., Bossis I., Gelasakis A. I. Serological, molecular and culture-based diagnosis of lentiviral infections in small ruminants. Viruses. 2021; 13 (9):1711. https://doi.org/10.3390/v13091711
13. Volkova I. Yu. Caprine arthritis-encephalitis epizootic monitoring and improvement of control measures in the Russian Federation: Author’s abstract of thesis for degree of Cand. Sci. (Veterinary Medicine). Pokrov; 2008. 25 p. (in Russ.)
14. Barsukov N. P. Cytology, histology, embryology. 6th ed., stereotypical. Saint Petersburg: Lan’; 2023. 268 p. (in Russ.)
15. Studentsov A. P., Shipilov V. S., Nikitin V. Ya., Petrov A. M., Dulger G. P., Khramtsov V. V., Preobrazhensky O. N. Animal obstetrics, gynecology and reproduction biotechnology: study guide for higher school. 12th ed., stereotypical. Saint Petersburg: Lan’; 2022. 548 p. (in Russ.)
16. Rodrigues A. de S., Pinheiro R. R., de Brito R. L. L., Oliveira L. S., de Oliveira E. L., dos Santos V. W. S., et al. Evaluation of caprine arthritis-encephalitis virus transmission in newborn goat kids. Arquivos do Instituto Biológico. 2017; 84:e0542016. https://doi.org/10.1590/1808-1657000542016
17. Furtado Araújo J., Andrioli A., Pinheiro R. R., Sider L. H., de Sousa A. L. M., de Azevedo D. A. A., et al. Vertical transmissibility of small ruminant lentivirus. PLoS ONE. 2020; 15 (11):e0239916. https://doi.org/10.1371/journal.pone.0239916
18. World Organisation for Animal Health. Terrestrial Animal Health Code. https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access
19. Poloz A. I., Finogenov A. Yu. Methodical guidelines for humane euthanasia of animals. Minsk: S. N. Vyshelessky Institute of Experimental Veterinary Medicine; 2008. 45 p. (in Russ.)
20. Koudryashov A. A. Pathoanatomical opening of corpses of animals. Part 1. Conditions of opening; the order of opening of corpses of different animals. Veterinarnaya praktika. 2004; (4): 27–31. https://elibrary.ru/yzuojo (in Russ.)
About the Authors
V. Yu. KoptevRussian Federation
Vyacheslav Yu. Koptev, Cand. Sci. (Veterinary Medicine), Leading Researcher, Head of the Laboratory of Diseases of Young Animals
Krasnoobsk 630501, Novosibirsk Oblast, Russia
N. A. Shkil
Russian Federation
Nikolay A. Shkil, Dr. Sci. (Veterinary Medicine), Professor, Chief Researcher, Laboratory of Diseases of Young Animals
Krasnoobsk 630501, Novosibirsk Oblast, Russia
N. Yu. Balybina
Russian Federation
Natalia Yu. Balybina, Researcher, Laboratory of Diseases of Young Animals
Krasnoobsk 630501, Novosibirsk Oblast, Russia
T. N. Belenkova
Russian Federation
Tatyana N. Belenkova, Company Specialist
18g Elektrikov str., Ekaterinburg 620091, Russia
Review
For citations:
Koptev V.Yu., Shkil N.A., Balybina N.Yu., Belenkova T.N. Study of possible intrauterine infection of goat fetus with caprine arthritis-encephalitis virus. Veterinary Science Today. 2024;13(4):338-343. https://doi.org/10.29326/2304-196X-2024-13-4-338-343



































