Scroll to:
Testing of vaccine against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies for its antigenic properties
https://doi.org/10.29326/2304-196X-2025-14-2-179-185
Abstract
Introduction. Recently “Carnican-5R” vaccine against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies has been developed at the Rosselkhoznadzor-subordinated Federal Center for Animal Health (FGBI “ARRIAH”, Vladimir) in accordance with the Russian Federation legislative requirements. The virus strains currently circulating and significant in the country were used for the vaccine development.
Objective. Testing of “Carnican-5R” vaccine for its antigenic properties in target animals including determination of humoral immunity development time and duration during the observation period.
Materials and methods. “Carnican-5R” combined vaccine containing two components: freeze-dried component and liquid component were used for the test. Dogs at the age of 10–12 weeks served as animal models for testing the vaccine for its antigenic properties. The antibody levels were determined with virus neutralization test, hemagglutination inhibition test and fluorescent antibody virus neutralization test.
Results. Vaccination of dogs was found to induce antibodies to the pathogens of the specified infections. Double “Carnican-5R” vaccine administration at 21-day interval induced strong humoral response by day 35 after its first administration and an increase in the antibody titers to canine distemper – by 8.6 times, to canine parvovirus type 2 – by 2.1 times, to canine coronavirus – by 5.0 times, to canine adenovirus serotype 2 – by 5.36 times, to the rabies virus – by 5.72 times. The specific immunity lasted for at least 12 months and virus-specific antibodies titers to the pathogens remained at the protective levels.
Conclusion. “Carnican-5R” vaccine is safe and non-reactogenic for target animals and induces strong immunity in dogs that lasts for at least 12 months from the date of booster vaccination.
Keywords
For citations:
Klimova A.A., Komarova A.A., Kiselev A.M., Galkina T.S. Testing of vaccine against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies for its antigenic properties. Veterinary Science Today. 2025;14(2):179-185. https://doi.org/10.29326/2304-196X-2025-14-2-179-185
INTRODUCTION
Canine distemper virus (CDV) affects some animal species of carnivorous order including canids, raccoons, felines, etc., as well as pandas. The pathogen is polytrophic and can affect almost all body systems. According to the international classification, the virus belongs to the order Mononegavirales, family Paramyxoviridae, subfamily Orthoparamyxovirinae, genus Morbillivirus, and species Morbillivirus canis [1][2].
Canine parvovirus type 2 (CPV-2) is the parvovirus enteritis agent and the main cause of dog fatalities associated with viral diseases; it is extremely contagious. Clinical signs are as follows: acute gastroenteritis, large intestine mucosal lining sloughing and hemorrhagic inflammation, hemorrhagic diarrhea, dehydration, leukopenia and neutropenia [2]. The virus belongs to the order Piccovirales, family Parvoviridae, subfamily Parvovirinae, genus Protoparvovirus and species Protoparvovirus carnivoran 1 [1].
Canine coronavirus (CCoV) causes enteritis with characteristic symptoms including anorexia, vomiting, diarrhea, lymphopenia, and lethargy [3-6]. The disease varies from asymptomatic to fatal [7]. Coronavirus ranks second position among viral enteropathogens in the world [8-10]. The pathogen belongs to the order Nidovirales, suborder Cornidovirineae, family Coronaviridae, subfamily Orthocoronavirinae, genus Alphacoronavirus [1].
Canine adenovirus serotype 2 (CAV-2) causes infectious laryngotracheitis in transient, asymptomatic or mild forms; it can cause severe necrotizing bronchitis, interstitial pneumonia [11], diarrhea [12] and central nervous system disorders [2]. The virus belongs to the order Rowavirales, family Adenoviridae, genus Mastadenovirus, species Mastadenovirus canidae, serotype 2 [1].
Rabies virus (RABV) affects the central nervous system of warm-blooded animals and humans and causes fatal disease [13][14]. There is no treatment for rabies. The virus belongs to the order Mononegavirales, family Rhabdoviridae, subfamily Alpharhabdovirinae and genus Lyssavirus [1][15][16].
The strains formulated in “Carnican-5R” combined vaccine against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies were selected according to the guidelines published by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA). According to the guidelines, canine distemper, canine parvovirus enteritis and canine adenovirus infection (CAV-2) are the major viral diseases that should be prevented regardless of the geographical location of the animal. The leptospirosis prevention with vaccines is classified as additional in the guidelines [17]. Rabies virus is included in the vaccine as anti-rabies immunization is envisaged by the Russian Federation legislation [18][19]. However, these guidelines are not mandatory, and vaccination schedules are developed by veterinarians taking into account occurrence of viral diseases in the particular region. In view of increasing number of deaths in the dog population due to coronavirus enteritis [5][6][10], the canine coronavirus strain responsible for this disease is also included in the vaccine composition.
Dog immunization schedule was developed based on scientific data on humoral immunity and the WSAVA recommendations. Generally, veterinarians around the world recommend to start vaccination of dogs at the age of 12 weeks, to perform booster vaccination at the age of 16 weeks, and then vaccinate the animal once a year or once every three years, depending on the viral animal disease situation in the region, during the whole animal life [17].
During “Carnican-5R” vaccine development, tests of the viruses formulated in the vaccine for their non-reactogenicity and safety were performed in laboratory animals in addition to the tests of the said viruses for their properties and determination of the proportions of the components formulated in the vaccine. Also, tests for selection of optimal vaccine immunizing dose and administration route were carried out. The tests have shown that the vaccine is nonreactogenic and safe, the immunizing dose is 1.0 cm3 (the liquid vaccine component serves as diluent for the freeze dried vaccine component), the vaccine is administrated by subcutaneous or intramuscular routes. The storage period after combining the components was 2 hours at a temperature of 18–25 °C.
For testing the vaccine for its antigenic properties, experiments were designed and carried out in target animals to study the humoral immunity development and the virus-specific antibody persistence time after the vaccine administration during the observation period (12 months).
The study was aimed at testing the vaccine against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies for its antigenic properties in target animals. As a result, high-quality immunobiological product compliant with specified parameters was prepared, test program was designed, tests including serological tests for determination of antibody levels before and after vaccination were carried out, obtained data were processed and structured.
MATERIALS AND METHODS
“Carnican-5R” vaccine against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies was tested for its antigenic properties in accordance with the requirements of Order No. 101 of the Ministry of Agriculture of the Russian Federation of 6 March 2018 on approval of the rules for veterinary medicinal product preclinical studies, clinical trials and bioequivalence studies.
Vaccine. “Carnican-5R” vaccine, developed by the Federal Centre for Animal Health consists of 2 components: freeze-dried vaccine component contains attenuated canine distemper virus and liquid vaccine component contains inactivated canine parvovirus, canine coronavirus, canine adenovirus and dog rabies virus. The vaccine has been registered in the Russian Federation, and the patent for the invention has been obtained [20].
The active substance of the freeze dried vaccine component is attenuated CDV Rockborn strain; the active substance of the liquid vaccine component is aminoethylethylenimine-inactivated CPV-2 Grey strain, CCoV Rich strain, CAV-2 Unity strain, RABV ARRIAH strain. The freeze dried vaccine component is supplemented with stabilizers: lactalbumin hydrolysate, sucrose and gelatose, the liquid vaccine components is supplemented with aluminum hydroxide as an adjuvant. All source materials used for the vaccine production have passed comprehensive incoming quality control. One immunizing dose of the vaccine contains at least 3.0 lg TCID50/cm3 of attenuated CDV and inactivated CPV-2 (virus titre before inactivation – at least 7.0 log2, HAU 1:128), CCoV (virus titre before inactivation – at least 3.0 lg TCID50/cm3), CAV-2 (virus titre before inactivation – at least 3.0 lg TCID50/cm3), RABV (virus titre before inactivation – at least 1.0 IU/cm3).
Animals. Clinical trials were carried out in 10–12 week-old dogs (n = 35).
The animals were kept in shelters, veterinary clinics and individually in private households. The health status of the dogs was assessed before the trial and during the whole observation period.
To test “Carnican-5R” vaccine for its effectiveness, puppies were immunized twice with a 21-day interval; the vaccine was injected at one immunizing dose intramuscularly into the caudal proximal area of hind leg.
All tests were carried out in accordance with the requirements of the following Federal Centre for Animal Health standards: STO 00495527-0002 “Laboratory animals used for tests and experiments” and STO 00495527-0230 “Preclinical studies of veterinary medicinal products”.
Serological tests. Sera from dogs were tested for antibodies to CDV, CCoV, CAV-2 with virus neutralization test (VNT) in microplates [21-23], for antibodies against RABV with fluorescent antibody virus neutralization test (FAVN test) and for antibodies against CPV-2 with hemagglutination inhibition test (HI test) according to the approved methodical guidelines [24].
Statistical analysis of the test results. The test results were processed using statistical methods in the Microsoft Excel program. Specific antibody titres were calculated using the Karber formula and expressed as log2.
RESULTS AND DISCUSSION
No body temperature changes, general physiological state deterioration, anorexia, and local reactions at the site of the vaccine injection were observed after vaccination. No signs of canine distemper, canine parvovirus and coronavirus enteritis, canine adenovirus infection and dog rabies were observed.
Tests of puppy sera showed that mean group specific antibody titres in sera collected before vaccination were as follows: mean group specific antibody titre against CDV, CCoV, CAV-2 was < 1.0 log2 (when sera were tested with VNT); against CPV-2 – 4.23 ± 0.63 log2 (when sera were tested with HI test); against RABV – < 0.5 log2 (when sera were tested with FAVN test).
Figure 1 shows the humoral immunity dynamics in dogs after “Carnican-5R” vaccine administration. Vaccination was found to induce virus-specific antibodies against CDV, CPV-2, CCoV, CAV-2 and RABV. The antibody levels 21 days after the first immunization were as follows: against CDV – 4.00 ± 0.25 log2; against CPV-2 – 5.67 ± 0.58 log2; against CCoV – 2.67 ± 0.14 log2; against CAV-2 – 2.83 ± 0.14 log2; against RABV – 0.82 ± 0.03 log2, and were significantly higher than threshold values.
Mean group virus-specific antibodies titres on day 7, 14, 21, and 35 were higher than the titres in dogs before their immunization. On day 35, virus-specific antibody level was significantly higher than that ones determined at previous test points (p ≥ 0.1) and was as follows: antibody titre against CDV – 8.60 ± 0.14 log2; against CPV-2 – 8.93 ± 0.58 log2; against CCoV – 5.0 ± 0.25 log2; against CAV-2 – 5.36 ± 0.14 log2; against RABV – 2.86 ± 0.07 log2. Antibody levels demonstrated a “plateau effect” on day 42 and day 51. Based on the data obtained, it was concluded that double administration of “Carnican-5R” vaccine at a 21-day interval induced strong humoral response by day 35 after the first vaccine administration. Thus, the following schedule for vaccination of dogs against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies was recommended: first vaccination – at the age of 10–12 weeks, booster vaccination – after 21 days and then annual vaccination. The vaccination schedule for adult animals is similar and does not depend on the age of the dog.
At the next stage, the immunity duration after double vaccination against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies was studied. For this purpose, blood samples were taken from the animals every 30 days for 12 months (observation period).
As shown in Figure 2, the duration of immunity to the pathogens of these diseases was at least 12 months. Slight decrease in the specific antibody levels was recorded at the time of booster vaccination, one year after the first vaccination of dogs. However, according to the published studies, the protective level of antibodies to CDV is 2–4 log2 [25][26]. Double vaccine administration induced an increase in antibodies up to 9.15 ± 0.14 log2, the minimum value was 7.75 ± 0.14 log2 during the observation period. The level of antibodies should be at least 4 log2 to protect dogs from parvovirus enteritis [25-31]. After double vaccination, the maximum level of antibodies to CPV-2 was 10.40 ± 0.58 log2, the minimum anti-CPV-2 antibody level was 9.0 ± 0.28 log2. Double immunization induced an increase in antibodies to CAV-2 up to 6.35 ± 0.25 log2. The minimum anti-CAV-2 antibody level was 5.90 ± 0.14 log2 during the observation period of 12 months. There are no reliable data on protective level of antibodies against CCoV, however, the anti-CCoV antibody titre was higher than 5.95 ± 0.14 log2 after the second vaccine administration, and the minimum anti-CCoV antibody titre was 5.08 ± 0.28 log2 suggesting that animals were protected from the disease [32]. According to scientific data and the requirements of the World Organization for Animal Health, the anti-rabies vaccine should induce anti-rabies virus antibodies at a titre of ≥ 0.5 IU/cm3 [14][33]. In our study, the maximum titre of antibodies against RABV was 3.69 IU/cm3, the minimum titre of antibodies against RABV was 2.6 IU/cm3 after immunization.
During the year, mean titre of antibodies against CDV was 8.74 ± 0.53 log2, against CPV-2 was 9.95 ± 0.42 log2, against CCoV was 5.75 ± 0.34 log2, against CAV-2 was 6.09 ± 0.14 log2, against RABV was 3.12 ± 0.37 IU/cm3. Thus, “Carnican-5R” vaccine induces humoral antibodies to canine distemper virus, canine parvovirus and coronavirus, canine adenovirus serotype 2 and rabies virus.
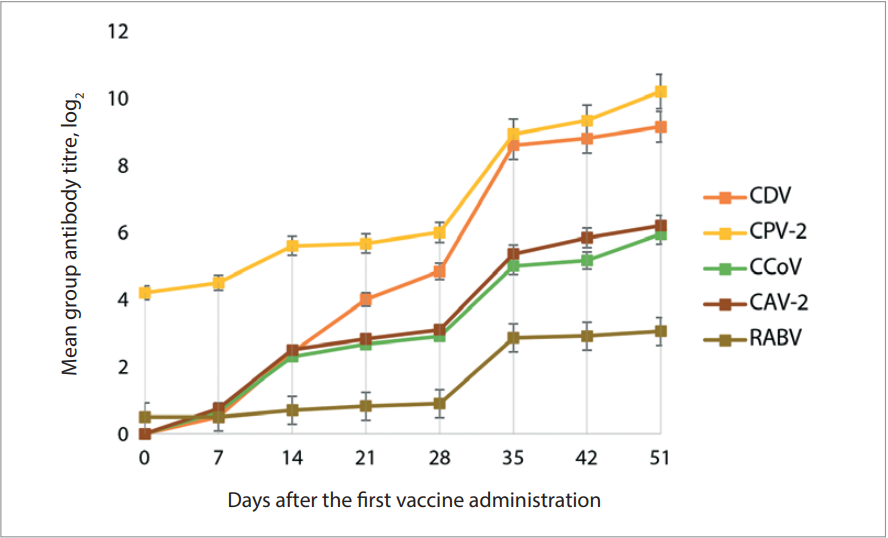
Fig. 1. Development of humoral immunity in dogs after “Carnican-5R” vaccine administration
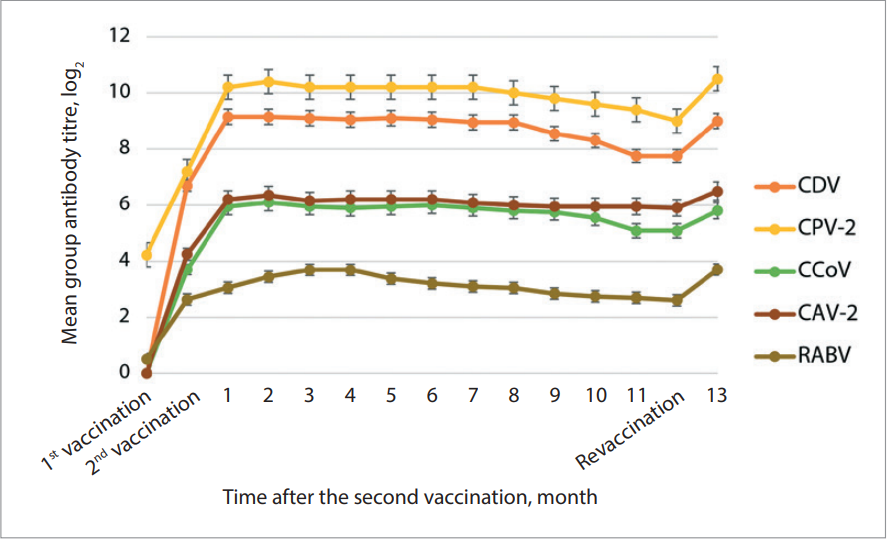
Fig. 2. Duration of the immunity in dogs after double “Carnican-5R” vaccine administration
CONCLUSION
During the study, the vaccine against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies was tested for its antigenic properties. Based on the data obtained, “Carnican-5R” vaccine was found to induce seroconversion in the target animals. The immunity lasts at least 12 months after double vaccine administration at a 21-day interval. The immune response develops 21 days after double vaccine administration. The mean antibody titres one month after booster vaccination were as follows: against CDV – 9.15 log2 (when tested with VNT), against CPV-2 – 10.2 log2 (when tested with HI test), against CCoV – 5.95 log2 (when sera were tested with VNT), against CAV-2 – 6.2 log2 (when sera were tested with VNT), against RABV – 3.05 IU/cm3 (when sera were tested with FAVN test). The level of antibodies to these viruses in dogs is higher than the protective level and protects the animal from these infections.
The vaccine is nonreactogenic and safe, does not cause any pronounced local reaction when administered intramuscularly or subcutaneously, the vaccine has no adverse effect on the physiological state of animals. The vaccine induces a pronounced immune response owing to production of virus-specific antibodies at protective titres.
“Carnican-5R” combined vaccine against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies developed at the Federal Centre for Animal Health can be used for specific prevention of the viral diseases in dogs. The vaccine has passed testing at the Russian State Center for Animal Feed and Drug Standardization and Quality and is registered in the Russian Federation.
References
1. International Committee on Taxonomy of Viruses: ICTV. https://ictv.global/taxonomy
2. Shevchenko A. A., Zerkalev D. Yu., Shevchenko L. V., Chernykh O. Yu., Gorpinchenko E. A. Infectious diseases of pet animals: study guide. Krasnodar: KubSAU; 2018. 107 р. (in Russ.)
3. Pratelli A., Tempesta M., Elia G., Martella V., Decaro N., Buonavoglia C. The knotty biology of canine coronavirus: a worrying model of coronaviruses’ danger. Research in Veterinary Science. 2022; 144: 190–195. https://doi.org/10.1016/j.rvsc.2021.11.014
4. Parkhe P., Verma S. Evolution, interspecies transmission, and zoonotic significance of animal coronaviruses. Frontiers in Veterinary Science. 2021; 8:719834. https://doi.org/10.3389/fvets.2021.719834
5. Decaro N., Buonavoglia C. An update on canine coronaviruses: viral evolution and pathobiology. Veterinary Microbiology. 2008; 132 (3–4): 221–234. https://doi.org/10.1016/j.vetmic.2008.06.007
6. Zappulli V., Caliari D., Cavicchioli L., Tinelli A., Castagnaro M. Systemic fatal type II coronavirus infection in a dog: pathological findings and immunohistochemistry. Research in Veterinary Science. 2008; 84 (2): 278–282. https://doi.org/10.1016/j.rvsc.2007.05.004
7. Licitra B. N., Duhamel G. E., Whittaker G. R. Canine enteric coronaviruses: emerging viral pathogens with distinct recombinant spike proteins. Viruses. 2014; 6 (8): 3363–3376. https://doi.org/10.3390/v6083363
8. Alves C. D. B. T., Granados O. F. O., Budaszewski R. D. F., Streck A. F., Weber M. N., Cibulski S. P., et al. Identification of enteric viruses circulating in a dog population with low vaccine coverage. Brazilian Journal of Microbiology. 2018; 49 (4): 790–794. https://doi.org/10.1016/j.bjm. 2018.02.006
9. McElligott S., Collins P. J., Sleator R. D., Martella V., Decaro N., Buonavoglia C., O’Shea H. Detection and genetic characterization of canine parvoviruses and coronaviruses in southern Ireland. Archives of Virology. 2011; 156 (3): 495–503. https://doi.org/10.1007/s00705-010-0861-3
10. Decaro N., Desario C., Billi M., Mari V., Elia G., Cavalli A., et al. Western European epidemiological survey for parvovirus and coronavirus infections in dogs. The Veterinary Journal. 2011; 187 (2): 195–199. https://doi.org/10.1016/j.tvjl.2009.10.027
11. Guidance on virology: human and animal viruses and viral infections. Ed. by D. K. Lvov. Moscow: Meditsinskoe informatsionnoe agentstvo; 2013. 1200 p. (in Russ.)
12. Koptopoulos G., Cornwell H. J. C. Canine adenoviruses: a review. Veterinary Bulletin. 1981; 51 (3): 135–142. https://www.cabidigitallibrary.org/doi/pdf/10.5555/19812282132
13. Gavrilov A. V., Zotova A. V. Rabies: study guide. Blagoveshchensk: Amur State Medical Academy; 2020. 38 p. (in Russ.)
14. Rabies (infection with rabies virus and other lyssaviruses). In: WOAH Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2023; Chapter 3.1.18. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.19_RABIES.pdf
15. Tordo N., Poch O. Structure of rabies virus. In: Rabies. Developments in Veterinary Virology. Ed. by J. B. Campbell, K. M. Charlton. Boston: Springer; 1988; 7: 25–45. https://doi.org/10.1007/978-1-4613-1755-5_2
16. Kuzmin I. V., Tordo N. Genus Lyssavirus. In: Rhabdoviruses: Molecular Taxonomy, Evolution, Genomics, Ecology, HostVector Interactions, Cytopathology and Control. Ed. by R. G. Dietzgen, I. V. Kuzmin. Norfolk: Caister Academic Press; 2012; Chapter 5: 37–57.
17. Squires R. A., Crawford C., Marcondes M., Whitley N. 2024 guidelines for the vaccination of dogs and cats – compiled by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association ( WSAVA). Journal of Small Animal Practice. 2024; 65 (5): 277–316. https://doi.org/10.1111/jsap.13718
18. Veterinary rules for preventive, diagnostic, restrictive and other measures, quarantine and other restriction imposition and lifting aimed at rabies spread prevention and outbreak eradication: approved by Order No. 705 of the Ministry of Agriculture of 25 November 2020 (as amended on 24 August 2021). https://base.garant.ru/75095535/#friends (in Russ.)
19. WSAVA and OIE call for action on rabies. Veterinary Record. 2013; 173 (19): 463–464. https://doi.org/10.1136/vr.f6792
20. Galkina T. S., Komarova A. A., Klimova A. A., Doronin M. I., Borisov A. V., Mikhalishin D. V. Associated vaccine against distemper, parvovirus and coronavirus enteritis, adenovirus infection and canine rabies. Patent No. 2817255 Russian Federation, Int. Cl. A61K 39/12 (2006.01), C12N 7/00 (2006.01), А61К 39/12 (2024.01), C12N 7/00(2024.01). Federal Centre for Animal Health. No. 2023115792. Date of filing: 15.06.2023. Date of publication: 12.04.2024. Bull. No. 11. (in Russ.)
21. Klimova A. A., Komarova A. A., Kiselev A. M., Galkina T. S. Methodical guidelines for canine adenovirus titration by micromethod: No. 35-22. Vladimir: Federal Centre for Animal Health; 2022. 22 p. (in Russ.)
22. Komarova A. A., Galkina T. S., Kiselev A. M., Klimova A. A. Methodical guidelines for canine enteric coronavirus titration by micromethod: No. 37-22. Vladimir: Federal Centre for Animal Health; 2022. 20 p. (in Russ.)
23. Kiselev A. M., Komarova A. A., Galkina T. S., Klimova A. A. Methodical guidelines for canine distemper virus titration by micromethod: No. 36-22. Vladimir: Federal Centre for Animal Health; 2022. 20 p. (in Russ.)
24. Klimova A. A., Komarova A. A., Kiselev A. M., Galkina T. S. Methodical guidelines for canine enteric parvovirus titration with hemagglutination tests using micromethod. No. 38-22. Vladimir: Federal Centre for Animal Health; 2022. 20 p. (in Russ.)
25. Böhm M., Herrtage M. E., Thompson H., Weir A., Hasted A. M., Maxwell N. S., Serum antibody titres to canine parvovirus, adenovirus and distemper virus in dogs in the UK which had not been vaccinated for at least three years. Veterinary Record. 2004; 154 (15): 457–463. https://doi.org/10.1136/vr.154.15.457
26. Gray L. K., Crawford P. C., Levy J. K., Dubovi E. J. Comparison of two assays for detection of antibodies against canine parvovirus and canine distemper virus in dogs admitted to a Florida animal shelter. Journal of the American Veterinary Medical Association. 2012; 240 (9): 1084–1087. https://doi.org/10.2460/javma.240.9.1084
27. Abdelmagid O. Y., Larson L., Payne L., Tubbs A., Wasmoen T., Schultz R. Evaluation of the efficacy and duration of immunity of a canine combination vaccine against virulent parvovirus, infectious canine hepatitis virus, and distemper virus experimental challenges. Veterinary Therapeutics. 2004; 5 (3): 173–186. https://www.researchgate.net/publication/8150149
28. Mouzin D. E., Lorenzen M. J., Haworth J. D., King V. L. Duration of serologic response to five viral antigens in dogs. Journal of the American Veterinary Medical Association. 2004; 224 (1): 55–60. https://doi.org/10.2460/javma.2004.224.55
29. Schultz R. D., Thiel B., Mukhtar E., Sharp P., Larson L. J. Age and long-term protective immunity in dogs and cats. Journal of Comparative Pathology. 2010; 142 (Suppl. 1): S102–S108. https://doi.org/10.1016/j.jcpa.2009.10.009
30. Schultz R. D. Duration of immunity for canine and feline vaccines: a review. Veterinary Microbiology. 2006; 117 (1): 75–79. https://doi.org/10.1016/j.vetmic.2006.04.013
31. Canine enteric viral infections. In: Sykes J. E., Greene C. E. Infectious diseases of the dog and cat. 4th ed. Elsevier; 2011; Chapter 8: 67–80.
32. Pratelli A., Tinelli A., Decaro N., Martella V., Camero M., Tempesta M., et al. Safety and efficacy of a modified-live canine coronavirus vaccine in dogs. Veterinary Microbiology. 2004; 99 (1): 43–49. https://doi.org/10.1016/j.vetmic.2003.07.009
33. Cliquet F., Aubert M., Sagné L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. Journal of Immunological Methods. 1998; 212 (1): 79–87. https://doi.org/10.1016/s0022-1759(97)00212-3
About the Authors
A. A. KlimovaRussian Federation
Anastasia A. Klimova, Veterinarian, Laboratory for Pets Disease Prevention,
Yur’evets, Vladimir 600901.
A. A. Komarova
Russian Federation
Anna A. Komarova, Leading Veterinarian, Laboratory for Pets Disease Prevention,
Yur’evets, Vladimir 600901.
A. M. Kiselev
Russian Federation
Alexey M. Kiselev, Veterinarian, Laboratory for Pets Disease Prevention,
Yur’evets, Vladimir 600901.
T. S. Galkina
Russian Federation
Tatyana S. Galkina, Cand. Sci. (Veterinary Medicine), Head of Laboratory for Pets Disease Prevention,
Yur’evets, Vladimir 600901.
Review
For citations:
Klimova A.A., Komarova A.A., Kiselev A.M., Galkina T.S. Testing of vaccine against canine distemper, parvovirus and coronavirus enteritis, adenovirus infection and dog rabies for its antigenic properties. Veterinary Science Today. 2025;14(2):179-185. https://doi.org/10.29326/2304-196X-2025-14-2-179-185



































