Scroll to:
Towards improved differential diagnostics of bovine tuberculosis in the Republic of Dagestan
https://doi.org/10.29326/2304-196X-2025-14-2-164-170
Abstract
Introduction. Non-specific tuberculin reactions are among the most critical challenges in tuberculosis diagnosis, with their incidence increasing annually. Given the complex epidemiological challenges, improving bovine tuberculosis diagnostics is critically important.
Objective. Development of effective comprehensive differential bovine tuberculosis diagnosis and introduction of improved techniques for the infection detection in farms with different animal health statuses in the Republic of Dagestan.
Materials and methods. 1,670 cattle were subjected to tuberculin testing; 3,502 serum samples were used for serological testing, 112 samples for immunological testing, 57 samples of pathological material collected from animals and 76 environmental samples were used for bacteriological testing. Mycobacterium bovis, Mycobacterium bovis BCG, Mycobacterium avium, Mycobacterium scrofulaceum strains were used in the study.
Results. Nonspecific reactions in the farms of all categories were found to be widespread in the Republic. Diagnostic value of intradermal and intravenous tuberculin tests in tuberculosis-infected herds was determined (9.4% of extra-detected diseased animals). Complement fixation test is poorly sensitive and highly specific. © Баратов М. О., 2025
Indirect haemagglutination assay results are not confirmed by conventional methods in most cases, which suggests their low specificity. 39 mycobacterial isolates were recovered from 57 biological samples and identified: 8 (20.5%) as M. bovis; 31 (79.5%) as non-tuberculous mycobacteria (acid-fast species), among them 29 (93.5%) were identified as Runyon II organisms, 2 (6.5%) as Runyon III organisms. 43 isolates out of 76 environmental samples were recovered: among them 2 (4.6%) were identified as Mycobacterium bovis, 23 (53.5%) as Runyon II organisms and 18 (41.9%) as Runyon III organisms. Among culture media, Löwenstein- Jensen’s egg-based medium provides the best growth performance and most effective suppression of competing microflora.
Conclusion. The obtained data provide a fundamental basis for developing an effective comprehensive method for differential diagnosis of bovine tuberculosis.
Keywords
For citations:
Baratov M.O. Towards improved differential diagnostics of bovine tuberculosis in the Republic of Dagestan. Veterinary Science Today. 2025;14(2):164-170. https://doi.org/10.29326/2304-196X-2025-14-2-164-170
INTRODUCTION
Animal tuberculosis control in the Caspian region, including in the Republic of Dagestan, has attained some success. At the same time, an analysis of the epizootic situation in this territory shows that the disease prevalence varies between the regions and the number of infected farms has remained almost unchanged in recent years [1-4].
Practice shows that the implementation of measures to prevent the infection introduction to free farms and to achieve freedom from the disease on the infected farms must be constantly monitored. Uncontrolled movements of livestock, animal products and feed pose risks of introducing the pathogen into disease-free farms. All these circumstances necessitate the need to constantly improve measures for the prevention and control of bovine tuberculosis (bTB), taking into account the changing epizootic situation and the peculiarities of modern animal husbandry [5][6].
One of the main issues in control system is qualified diagnostics, which often requires complex and targeted tests that are not covered by current regulations [7][8].
As the incidence of animal tuberculosis declines, the issue of non-specific reactions unrelated to the disease is becoming increasingly pressing. At the same time, due to imperfect differentiation methods, such reactions bring great economic losses leading to slaughter of healthy livestock and costly animal health actions [9][10].
Since many aspects of such reaction mechanisms remain understudied, multiplicity of concepts still exists [11][12].
Despite extensive study, the etiology of nonspecific PPD-tuberculin reactions in animals has yet to be fully elucidated. Domestic and foreign literature data show that the main cause of nonspecific reactions in healthy animals are non-tuberculous mycobacteria and acid-resistant actinomycetes, which are morphologically, culturally, physiologically and genetically closely related to mycobacteria [13][14].
There are reports that mycobacterium-like organisms (Corynebacterium, Nocardia, Rhodococcus), which share group-specific features with mycobacteria, may also sensitize the animal body to tuberculin [3][4][15-17].
However, it has been established that not all tuberculosis-infected animals react to the tuberculin test. It is also known that when animals are tested using various methods, only reactors to certain tests are identified, which is probably due to chronic multi-stage disease duration, environmental factors, physiological state of the animal, etc. [18-21].
This certainly makes it difficult to diagnose tuberculosis and necessitates the use of a set of diagnostic tests, including tuberculin, serological, bacteriological and immunological ones. Each of these methods has advantages along with disadvantages, which increases the effectiveness of diagnosis [22-24].
It should be noted that a single diagnostic algorithm has not been developed yet, moreover, the role of serological and immunological methods, in our opinion, is often underestimated [25-27].
In this regard, further study of sensitization problems, spread of mycobacteria and related microorganisms in nature, their isolation rates from the biological samples from tuberculin-reacting animals and environmental samples, the ability to sensitize the body to tuberculin and their epizootic significance are of great scientific and practical value.
MATERIALS AND METHODS
The nature of allergic reactions was confirmed by intradermal and simultaneous tests using PPD-tuberculin for mammals and nontuberculous mycobacteria (NTM) complex in accordance with the “Veterinary rules for preventive, diagnostic, restrictive and other measures, the establishment and lifting of quarantine and other restrictions aimed to prevent and eradicate tuberculosis”1, which has been effective since March 1, 2021. A total of 1,670 cattle of different age groups (cows, heifers) have been tested.
A comparative analysis of different test results was performed: based on tuberculin intradermal, intravenous, intrapalpebral, and ophthalmic tests for allergic reactions on bTB-infected cattle farms – 170 animals; simultaneous and intrapalpebral tests on bTB free farms – 386 animals; serological tests: CFT with MAC (complement fixation test with mycobacterial antigen complex) – 1,411 serum samples; IHA (indirect hemagglutination) test using RBC diagnostic reagents – 2,091 samples; immunological tests: rosette test, LTT (lymphocyte transformation test), LSLT (leukocyte specific lysis test) – 112 samples; bacteriological tests – 57 biological samples and 76 environmental samples.
The collection of pathological material (blood, lesions, lymph nodes), transportation, storage, pre-culture treatment, preparation of nutrient media were performed in compliance with “Handbook of Microbiological and Virological Tests” (ed. by M. O. Birger, 1982; in Russ.).
Mycobacterium bovis, Mycobacterium bovis BCG, Mycobacterium avium, Mycobacterium scrofulaceum field strain cultures isolated from homogenated pathological material and environmental samples and collection strains of these cultures stored in the laboratory were used in the study.
Biological samples from tuberculin-reacting cattle of bTB-infected farms were handled according to Alikayeva’s method.
Pieces of pathological material were ground in a porcelain mortar with crushed glass. Then the homogenated material was poured into sterile vials with a 3% solution of C12H25SO4Na (sodium lauryl sulfate) in 1:1 ratio. After mixing, it was left at room temperature for 20 minutes. Then the vials were centrifuged for 20 minutes at 1,500 rpm, the supernatant was removed, the precipitate was washed twice with sterile distilled water and inoculated into Löwenstein – Jensen’s, Finn II, Petraniani, Gelberg media and modified Shkolnikova medium to detect and exclude cell wall deficient mycobacteria (L-forms).
Environmental samples (hay, straw, scrapings from feeders, soil, manure) were crushed, mixed with saline solution, ground and dispensed into vials with a 5% solution of H2SO4 (sulfuric acid) in 1:1 ratio and left at room temperature for 30 minutes. Then the vials were centrifuged at 1,500 rpm for 20 minutes, the supernatant was removed, the precipitate was washed twice by centrifugation and used for inoculation. Each sample of the preciptate was inoculated in 8 tubes and incubated in a thermostat at 37–38 °C.
Tuberculosis agents and non-tuberculous mycobacteria were differentiated in accordance with GOST 26072-89 (ST SEV 3457-81) “Livestock and poultry. Methods of tuberculosis laboratory diagnosis”2 and GOST 27318-87 (ST SEV 5627-86) “Livestock. Methods of nontuberculous mycobacteria identification”3.
All experiments were carried out in strict accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS No. 123).
RESULTS AND DISCUSSION
An analysis of the test results for allergic reactions indicates that reactions to PPD-tuberculin for mammals in the Republic of Dagestan are prevalent, regardless of the natural and climatic zones.
The ratio of animal reactors in all categories of farms was 30.9%, with a majority of reactions occurring on bTB free farms, which is indicative of widespread nonspecific reactions in healthy cattle.
It should be noted that the number of tuberculin reactors on free farms in mountainous and foothill areas is slightly less than on lowland farms (Table 1).
These figures significantly differ from previously published data, reflecting the correlation between the number of tuberculin reactors and vertical zonation. Comparative cartographic analysis shows that in the second half of the last century and in the beginning of the current century, the correlation between the number of both tuberculin-reactors and confirmed bTB cases and the number and density of animal populations and proximity to the lowland zone was recorded.
Analysis revealed no climate zone-dependent variations in tuberculin test reactivity or confirmed bTB incidence. The prevalence of reactors (45) and bTB cases (2) on mountainous zone farms (4.44% ratio), despite conditions favoring stronger immunity, appears attributable to: uncontrolled inter-farm interactions, substantial seasonal livestock transfers, and regular import of feed from lowlands.
While no bTB cases were confirmed among foothill zone reactors, the historical prevalence matches that of the lowland zone, precluding any conclusion of disease-free status. The data provided should be considered interim and require annual confirmation.
Tuberculin reactivity in cattle shows significant seasonal variation, with peak rates occurring during spring and autumn months. More than 80% of the reactors are detected during this period.
In order to compare the effectiveness of various tests for allergic reactions on bTB infected farms, 170 animals were tested with tuberculin intradermal, intravenous, intrapalpebral and ophtalmic tests (Fig. 1).
The diagnostic value of intradermal and intravenous tests and their role in bTB diagnosis were determined. It was established that most effective diagnostic approach for the animals from bTB infected herds is the combination of intradermal and intravenous tuberculin tests. The percentage of additionally detected diseased animals was 9.4%. This method proved to be effective both for the initial diagnosis and for the differentiation of tuberculin nonspecific reactions.
The comparative study of simultaneous and intrapalpebral tests of animals from bTB free farms for the purposes of initial diagnosis (386 animals were tested) gave inconclusivee results (Fig. 2): simultaneous test showed 19 PPD-tuberculin reacting animals (4.9%), 15 animals reacted to NTM complex (3.9%); 4 animals showed reactions to intrapalpebral administration of tuberculin (1.0%).
With Dagestan accounting for 21.3% of Russia’s sheep and goat population (ranking first) and 5.3% of cattle (ranking third), predominantly in backyard farming systems, developing reliable methods to differentiate non-specific reactions has become a critical modern priority. The standard simultaneous testing protocol proved ineffective under these conditions, while current regulations prohibit diagnostic slaughter as follow-up to skin test results. Studies revealed that the intravenous diagnostic method proved most effective under these conditions, showing particular advantages when testing small herds in private ownership settings
In animals displaying positive reactions to both intradermal and intravenous tests, post-mortem and laboratory analyses confirmed tuberculosis in 95.8% of cases, as demonstrated by comparative evaluation. In addition, in herds with long-term tuberculosis history, an intravenous test additionally reveals tuberculin-anergic animals, with their number ranging from 0.2 to 0.7%, according to numerous reports.
Detection rates of intradermal test reactors were assessed among stable / pasture-kept animals in backyard farming systems of two foothill settlements (Karabudakhkentsky and Buinaksky Raions, 167 animals) and on different farms in the lowland (SPK “Lvovskoye” Babayurtsky Raion, 123 animals, farm “Telmana” Kizlyarsky Raion, 274 animals). The results are shown in Figure 3.
Table 1
Percentage ratio of tuberculin reactors and tuberculosis-infected cattle in the Republic of Dagestan in 2022–2023
|
Area |
Cattle tested, animals |
Tuberculin reactors |
Diseased cattle identified, animals |
Reactors / diseased animals, % |
|
|
animals |
% |
||||
|
Mountainous |
167 |
45 |
26.9 |
2 |
4.44 |
|
Foothill |
182 |
59 |
32.4 |
– |
– |
|
Lowland |
201 |
66 |
32.8 |
4 |
6.06 |
|
Total |
550 |
170 |
30.9 |
6 |
3.50 |

Fig. 1. Assessment of diagnostic value of tuberculin tests in tuberculosis-infected farms
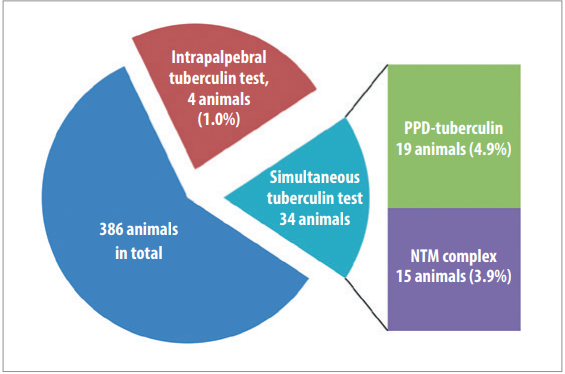
Fig. 2. Results of comparative analysis of simultaneous and intrapalpebral tests

Fig. 3. Number of tuberculin reactors raised in stall / pasture conditions
It was found that in all farms, except for “Telmana” farm in the Kizlyarsky Raion, more than 10% of cattle reacted to PPD-tuberculin.
The analysis of serological test results was based on the parallel use of tuberculin tests: 1,411 serum samples using CFT with MAC and 2,091 samples using IHA test with three RBC diagnostic reagents (M. bovis, M. avium, M. fortuitum). No reliable correlation was revealed between the number of intradermal test reactors and the number of animals demonstrating diagnostic antibody titers in their sera by serological tests.
The CFT demonstrates high specificity (85–100%) but low sensitivity, making it valuable for confirmatory diagnosis CFT shows promise in identification of anergic animals in chronically infected herds. Our observations show that the number of such animals in some farms can reach up to 10%.
The results of the highly sensitive IHA test using RBC diagnostic reagents in most cases are not confirmed by conventional methods (post-mortem and laboratory tests), which is indicative of its low specificity for tuberculosis. This test using several diagnostic reagents can serve as an additional test for tuberculin non-specific reaction differentiation.
Cellular immunity tests (rosette test, LTT, LSLT) exhibit appropriate sensitivity and specificity characteristics, but their complexity confines them primarily to research applications rather than mass screening programs.
The results of bacteriological test revealed that M. bovis can be isolated in pure culture in almost all biological samples from animals demonstrating bTB-consistent changes and from about 6% of asymptomatic animals.
Thirty nine mycobacterium cultures were isolated and identified from 57 biological samples: 8 (20.5%) were identified as M. bovis; 31 (79.5%) were non-tuberculous acid-resistant species, among them 29 (93.5%) were identified as Runyon II organisms and 2 (6.5%) as Runyon III organisms.
Seventy six environmental samples were collected; 43 isolates were recovered of which 2 (4.6%) were identified as M. bovis, 23 (53.5%) as Runyon II organisms, and 18 (41.9%) as Runyon III organisms (Table 2).
Isolation rates of certain Mycobacterium species differed significantly among sample types and disease progression stages. M. bovis isolation frequency decreased significantly from 40% during active disease progression to 14% in the fading stage. Similar changes were observed for non-tuberculous mycobacteria.
The correlation between the frequency and NTM isolation rate from biological and environmental samples can be traced in all natural and climatic zones.
The growth performance of various nutrient media was tested by inoculation of 34 mycobacterial cultures: 7 (20.6%) were identified as M. bovis, 27 (79.4%) as non-tuberculosis acid-resistant species, among them 11 (40.7%) were identified as Runyon II organisms and 16 (59.3%) as Runyon III organisms. The isolation rate was estimated by the number of colonies and the growth rate (Table 3).
A noticeable growth of both M. bovis (15 colonies without growth of foreign microflora in 17–19 days) and nontuberculous mycobacteria (19 M. avium colonies in 8 days and 16 colonies of M. sсrofulaceum in 7 days) was recorded on Löwenstein – Jensen's medium. Finn II medium performance was slightly poorer in both growth rates and colony counts (9 small M. bovis colonies in 17 days; 6, 17, and 13 M. bovis BCG, M. avium, and M. scrofulaceum colonies, respectively, in 6–11 days). Other media supported slow bacterial growth, producing only small colonies.
Table 2
Number of isolates from environmental and biological samples
|
Samples |
Total |
Isolated |
Identified |
|||||
|
M. bovis |
NTM |
|||||||
|
Runyon II organisms |
Runyon III organisms |
|||||||
|
No. |
% |
No. |
% |
No. |
% |
|||
|
Biological samples |
57 |
39 |
8 |
20.5 |
29 |
93.5 |
2 |
6.5 |
|
Environmental samples |
76 |
43 |
2 |
4.6 |
23 |
53.5 |
18 |
41.9 |
Table 3
Isolation rates for different nutrient media
|
Growth medium |
M. bovis |
M. bovis BCG |
М. avium |
M. scrofulaceum |
||||
|
Number of colonies |
Growth rate, days |
Number of colonies |
Growth rate, days |
Number of colonies |
Growth rate, days |
Number of colonies |
Growth rate, days |
|
|
Löwenstein –Jensen’s |
15 |
17–19 |
10 |
8 |
19 |
8 |
16 |
7 |
|
Finn II |
9 |
17 |
6 |
7 |
17 |
11 |
13 |
6 |
|
Petraniani |
5 |
20 |
7 |
6 |
10 |
12 |
8 |
10 |
|
Gelberg |
6 |
24 |
3 |
8 |
6 |
10 |
4 |
8 |
CONCLUSIONS
- The effectiveness of a set of differential diagnostic tests, including intrapalpebral, intravenous and intradermal tuberculin tests was demonstrated. The combined use of these tests identify diseased animals in bTB-infected herds and differentiate nonspecific reactions to PPD-tuberculin for mammals.
- Studies conducted over the past 3 years demonstrate high prevalence of tuberculin reactors, reaching 18% on some farms. In most cases, the nature of these reactions remains unclear.
- The analysis of serological results revealed CFT high specificity for tuberculosis diagnosis. We consider it reasonable to use this test as an additional method, in particular to identifiy animals anergic to tuberculin. Although serological (IHA) and immunological (rosette test, LTT, LSLT) tests are not yet widely adopted for diagnosing animal tuberculosis, they remain scientifically valuable.
- A comparative study of growth media most commonly used in the laboratory revealed that Löwenstein – Jensen's egg-based medium provides the best growth performance and isolation rates for both typical and nontuberculous mycobacteria. Despite its overall poorer performance, Finn II medium sometimes exhibits faster growth rates compared to Löwenstein – Jensen's medium.
1. https://docs.cntd.ru/document/565721619 (in Russ.)
2. https://docs.cntd.ru/document/1200025492 (in Russ.)
3. https://docs.cntd.ru/document/1200025497 (in Russ.)
References
1. Baratov M. O., Sakidibirov O. P., Akhmedov M. M. Epizooticheskie osobennosti tuberkuleza krupnogo rogatogo skota = Epizootic peculiarities of bovine tuberculosis. Ekologicheskie problemy sel’skogo khozyaistva i nauchno prakticheskie puti ikh resheniya: sbornik nauchnykh trudov Mezhdu narodnoi nauchnoprakticheskoi konferentsii (Makhachkala, 5–6 iyunya 2017 g.) = Environmental issues in agriculture and scientific and practical solutions: proceedings of the International Scientific and Practical Conference (Makhachkala, June 5–6, 2017). Makhachkala: Dagestan State Agrarian University; 2017; 102–108. https://elibrary.ru/zgksmn (in Russ.)
2. Baratov M. O. Improvement of bovine tuberculosis diagnosis. Veterinary Science Today. 2020; (4): 261–265. https://doi.org/10.29326/2304196X-2020-4-35-261-265
3. Baratov M. O., Sakidibirov O. P. Cattle tuberculosis in Dagestan Republic: problems and prospects. Veterinariya. 2021; (1): 24–28. https://doi.org/10.30896/0042-4846.2021.24.1.24-28 (in Russ.)
4. Nuratinov R. A., Gazimagomedov M. G. Tuberculosis. Makhachkala: Planeta-Dagestan; 2009. 336 p. (in Russ.)
5. Gulyukin M. I., Naymanov A. Kh., Ovdienko N. P., Vedernikov V. A., Verkh ovsky O. A., Tolstenko N. G., et al. Methodical instructions for conducting studies in animal mycobacteriosis. Moscow: All-Russian Research Institute of Experimental Vеterinary. 2012. 85 p. (in Russ.)
6. Donchenko A. S., Ovdienko N. P., Donchenko N. A. Diagnosis of bovine tuberculosis. Novosibirsk: Siberian Branch of the Russian Academy of Sciences; 2004. 308 p. (in Russ.)
7. Naimanov A. Kh., Ovdienko N. P., Pomykanov N. P. Diagnostika tuberkuleza krupnogo rogatogo skota v individual’nykh khozyaistvakh = Bovine tuberculosis diagnosis on individual farms. Aktual’nye problemy infektsionnoi patologii i immunologii zhivotnykh: materialy konferentsii (Moskva, 16–17 maya 2006 g.) = Topical issues of animal infectious pathology and immunology: proceedings of the conference (Moscow, 16–17 May 2006). Moscow: Izograf; 2006; 297–302. https://elibrary.ru/vyftgj (in Russ.)
8. Kamalieva Yu. R. Retrospective analysis of frequency of the occurrence of non-specific reactions to tuberculin in cattle in the Republic of Tatarstan. Molodezhnye razrabotki i innovatsii v reshenii prioritetnykh zadach APK: materialy Mezhdunarodnoy nauchnoy konferentsii = Young scientists’ development and innovations to solve priority agrarian tasks: Proceedings of the International Scientific Conference. Vol. 1. Kazan: Kazan State Academy of Veterinary Medicine; 2020; 278–280. https://elibrary.ru/vwhcco (in Russ.)
9. Baratov M. O., Huseynova P. S. More on search for causes of sensitization to tuberculin PPD for mammals in cattle. Veterinary Science Today. 2021; 10 (4): 271–276. https://doi.org/10.29326/2304-196X-2021-10-4-271-276
10. Dorozhko V. P. Specific stimulation during serological diagnosis of bovine tuberculosis: Author’s abstract of thesis for degree of Cand. Sci. (Veterinary Medicine). Kyiv; 1971. 20 p. (in Russ.)
11. Mukovnin A. A., Naimanov A. H., Gulukin A. M. Bovine tuberculosis in the Russian Federation. Veterinariya. 2020; (7): 19–24. https://doi.org/10.30896/0042-4846.2020.23.7.19-24 (in Russ.)
12. Mingaleev D. N. Novel tools and methods of bovine tuberculosis prevention in young animals: Author’s abstract of thesis for degree of Dr. Sci. (Veterinary Medicine). Kazan’; 2018. 42 p. (in Russ.)
13. Ionina S. V., Donchenko N. A., Donchenko A. S. The relationship between the circulation of atypical mycobacteria in the environment with the manifestation of tuberculin reactions in selskohozaystvennih. Innovations and Food Safety. 2016; (1): 41–44. https://doi.org/10.31677/2311-06512016-0-1-41-44 (in Russ.)
14. Bokova T. V. Frequency of non-specific reactions to PPD tuberculin in BLV infected cattle, and development of leucosis eradication programmes for breeding herds in the Altay Krai: Author’s abstract of thesis for degree of Cand. Sci. (Veterinary Medicine). Barnaul; 2001. 27 p. (in Russ.)
15. Azuma I., Ajisaka M., Yamamura Y. Polysaccharides of Mycobacterium bovis Ushi 10, Mycobacterium smegmatis, Mycobacterium phlei, and atypical Mycobacterium P1. Infection and Immunity. 1970; 2 (3): 347–349. https://doi.org/10.1128/iai.2.3.347-349.1970
16. Harriff M. J., Cansler M. E., Toren K. G., Canfield E. T., Kwak S., Gold M. C., Lewinsohn D. M. Human lung epithelial cells contain Mycobacterium tuberculosis in a late endosomal vacuole and are efficiently recognized by CD8+ T cells. PLoS ONE. 2014; 9 (5):e97515. https://doi.org/10.1371/journal.pone.0097515
17. Monin L., Griffiths K. L., Slight S., Lin Y., Rangel-Moreno J., Khader S. A. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunology. 2015; 8 (5): 1099–1109. https://doi.org/10.1038/mi.2014.136
18. Vlasenko V. S. Optimization of immunity control and correction methods during bovine tuberculosis and leucosis: Author’s abstract of thesis for degree of Dr. Sci. (Biology). Kazan; 2011. 43 p. (in Russ.)
19. Protodyakonova G. P. Epizootological and epidemiological peculiarities of tuberculosis in Yakutia, improvement of diagnosis and prevention methods: Author’s abstract of thesis for degree of Dr. Sci. (Veterinary Medicine). Novosibirsk; 2015. 35 p. (in Russ.)
20. Carneiro P. A. M., de Moura Sousa E., Viana R. B., Monteiro B. M., Do Socorro Lima Kzam A., de Souza D. C., et al. Study on supplemental test to improve the detection of bovine tuberculosis in individual animals and herds. BMC Veterinary Research. 2021; 17:137. https://doi.org/10.1186/s12917-021-02839-4
21. Khairullah A. R., Moses I. B., Kusala M. K. J., Tyasningsih W., Ayuti S. R., Rantam F. A., et al. Unveiling insights into bovine tuberculosis: a comprehensive review. Open Veterinary Journal. 2024; 14 (6): 1330–1344. https://doi.org/10.5455/OVJ.2024.v14.i6.2
22. Petrov R. V., Khaitov R. M. The bases of immunity and immune biotechnology. Annals of the Russian Academy of Medical Sciences. 2000; (11): 18–21. https://pubmed.ncbi.nlm.nih.gov/11186283 (in Russ.)
23. Tizard I. R. Veterinary Immunology: An Introduction. 6th ed. Philadelphia: Saunders; 2000. 482 p.
24. Ramos D. F., Silva P. E., Dellagostin O. A. Diagnosis of bovine tuberculosis: review of main techniques. Brazilian Journal of Biology. 2015; 75 (4): 830–837. https://doi.org/10.1590/1519-6984.23613
25. Barksdale L., Kim K. S. Mycobacterium. Bacteriological Reviews. 1977; 41 (1): 217–372. https://doi.org/10.1128/br.41.1.217-372.1977
26. Radchenkov V. P., Sokolovskaya I. I. Rosetting lymphocytes of cattle and rational methods for their detection. Agricultural Biology. 1983; (12): 87–91. (in Russ.)
27. Dzhupina S. I. Fundamental’nye znaniya epizooticheskogo protsessa – osnova kontrolya tuberkuleza krupnogo rogatogo skota = Fundamental knowledge of the epidemic process – the basis of bovine tuberculosis control. Russian Journal of Veterinary Pathology. 2004; (1–2): 45–47. https://elibrary.ru/hsowdp (in Russ.)
About the Author
M. O. BaratovRussian Federation
Magomed O. Baratov, Dr. Sci. (Veterinary Medicine), Chief Researcher, Head of Laboratory of Infectious Pathology of Farm Animals,
88, Dakhadaeva str., Makhachkala 367000, Republic of Dagestan.
Review
For citations:
Baratov M.O. Towards improved differential diagnostics of bovine tuberculosis in the Republic of Dagestan. Veterinary Science Today. 2025;14(2):164-170. https://doi.org/10.29326/2304-196X-2025-14-2-164-170



































