Scroll to:
Virucidal activity of disinfectants against African swine fever virus
https://doi.org/10.29326/2304-196X-2025-14-2-156-163
Abstract
Introduction. The most effective strategy to control African swine fever is to implement a set of anti-epizootic measures aimed at preventing introduction and spread of the disease pathogen. Currently, there is a wide range of commercially available disinfectants used at the facilities subject to veterinary control. Their effectiveness against African swine fever virus is unknown and is only confirmed by the manufacturers, who do not always provide substantiated evidence.
Objective. The objective of the research is to test virucidal activity of various disinfectants against African swine fever pathogen in the laboratory.
Materials and methods. Twelve samples of disinfectants with different chemical compositions were tested. The first in vitro assessment stage was carried out using suspension method, i.e. working solutions of the tested disinfectants in experimental concentrations and exposure times were added to the liquid-phase virus-containing material. During the second stage, swabs from concrete test plates contaminated with African swine fever virus were tested following treatment of surfaces with the working disinfectant solutions. Each stage was performed in two variants: without organic contamination and with its imitation (application of inactivated bovine serum on the test surface). The samples were tested using virus isolation in a sensitive porcine spleen cell culture. Results were assessed and interpreted in hemadsorption test. The disinfectant sample was considered to exhibit virucidal activity, if no reproduction of African swine fever virus was observed.
Results. Nine out of twelve tested disinfectants demonstrated a virucidal effect against reference African swine fever virus Arm 07 strain (genotype II), when tested on test surfaces. Such results suggest the need to evaluate further the efficacy of various disinfectants against this pathogen.
Conclusion. The fact that such disinfectant products that are incapable of inactivating African swine fever virus under the conditions specified in their instructions are potentially marketed underlines the need to improve regulatory framework in order to ensure effectiveness of general disease prevention and control measures.
Keywords
For citations:
Lavrentiev I.A., Igolkin A.S., Shevtsov A.А., Kolbin I.S., Puzankova O.S., Gavrilova V.L., Chernyshev R.S. Virucidal activity of disinfectants against African swine fever virus. Veterinary Science Today. 2025;14(2):156-163. https://doi.org/10.29326/2304-196X-2025-14-2-156-163
INTRODUCTION
Despite efforts taken to prevent spread of African swine fever (ASF) in the Russian Federation, there are still high risks to introduce the infection into pig farms. The damage caused by the disease is significant. As a result, Russia’s total losses from ASF in 2018–2020 amounted to 32,571.6 million rubles [1].
Under the current conditions, it is especially important to ensure biological security for all categories of pig farming enterprises, i.e. a set of administrative and physical measures taken to reduce the risk of the disease introduction, its rooting and spread [2, 3].
Failure to comply with or imperfect biosecurity regulations increase the likelihood of introducing pathogens of dangerous diseases, including African swine fever virus (ASFV). Therefore, it is important to implement effective protective measures (i.e. segregation, cleaning, disinfection) on all pig farms, pig slaughterhouses and pig processing plants, taking into account the possibility of virus introduction via virus sources (infected animals) and contaminated objects (vehicles, personnel attire, consumables, and farm equipment).
Relevant veterinary rules are implemented in case of ASF to define the structure of veterinary posts that shall treat vehicles at the exit from the outbreak and the protection zone, and shall conduct three-stage disinfection in the outbreak to prevent the infection spread [4]. As point 49 of the mentioned rules reads, that chlorine-containing disinfectants (minimum 25% active chlorine) or equivalent disinfecting agents with high virucidal activity against the pathogen shall be used for disinfection, according to the instructions for use. However, not all instructions for commercial disinfectants provide evidence-based data on their efficacy against target pathogens and proper application protocols.
All of the above indicates that in order to ensure protection against introduction and spread of ASF, effective disinfection measures are required that take into account the pathogen stability in the environment and pig products, as well as the virus tolerance to certain types of disinfectants resulting from a complex virion structure.
The temperature range of ASFV resistance is 5 °C – up to 7 years, 18–20 °C – 18 months, 37 °C – 30 days, 50 °C – up to 1 hour. The virus can persist for 6 years in serum at a temperature of 5 °C, in smoked ham – up to 180 days, in frozen meat – up to 155 days [5][6].
Data on survival of ASFV in external environments and in various excreta from the infected animals are given in Table 1.
Table 1
ASFV resistance to the environmental factors, based on findings from independent researchers
|
Environmental object |
Storage conditions |
Observation period |
Reference |
|
Faeces |
+4 °C |
5–280 days |
[7][8] |
|
+20 °C |
3–11 days |
[7][9] |
|
|
Manure |
–20 °C |
2 months |
[10] |
|
+4 °C |
30–145 days |
[8][10] |
|
|
+20 °C |
21 days |
[10] |
|
|
Urine |
–20 °C |
3 months |
[10] |
|
+4 °C |
5–60 days |
[7][10] |
|
|
+20 °C |
5–21 days |
[7][10] |
|
|
Beach sand |
+20 °C |
14 days |
[7] |
|
Backyard soil |
+20 °C |
7 days |
[7] |
|
Bog mud |
+20 °C |
3 days |
[7] |
|
Soil |
–20 °C |
2 months |
[10] |
|
+4 °C |
45–650 days |
[8][10] |
|
|
+20 °C |
30–132 days |
[8][10] |
|
|
Moist soil |
+4 °C |
up to 3 days |
[7] |
|
+20 °C |
up to 3 days |
[7] |
|
|
Water |
–20 °C |
3 years |
[11] |
|
+4 °C |
2–33 months |
[8][10][11] |
|
|
+20 °C |
2–13 months |
[8][10][11] |
It should be noted that ASFV is not mentioned in SanPiN 3.3686-21, since it does not belong to zoonotic pathogens [12]. Pursuant to point 2.13.2 of the “Rules for disinfection and decontamination at the facilities under state veterinary surveillance”, ASFV is classified as stable (stability group 2 out of four groups specified in the Rules) [13]. The document also includes disinfection requirements based on the pathogen stability characteristics. However, the listed disinfectants do not include a wide range of currently used products (based on acids, alkalis, aldehydes, chlorine compounds, iodine, phenols, quaternary ammonium compounds) with the following claimed advantages: relatively short exposure time, absence of pronounced corrosive and toxic effects, enhanced effect due to the synergy of components at low concentrations of active substances [14-16].
As specified in “Methodological guidelines for quality control of veterinary disinfection in livestock facilities” (see Appendix 3 to the above mentioned rules), in order to control the quality of the on-going disinfection, tests are specified for indicator microorganisms (for stability group 2: Staphylococcus aureus, S. epidermatis, S. saprophiticus) [13]. Although stability of ASFV and staphylococci is comparable, it is not equivalent, due to the difference in the pathogen structure and the specific mechanisms of resistance [17][18]. For example, key components of the cell wall structure in Gram-positive bacteria, including S. aureus, are peptidoglycan and teichoic acids, while the ASFV virion core is surrounded by a dense protein layer, an inner lipid envelope, and a capsid, which serves as the outer layer in intracellular virions. The aforementioned factors also contribute to the differences in sensitivity to various pH levels. For example, the optimal range for staphylococcal growth is between 7.2 and 7.4, and in case of high acidity (pH < 4.5), bacterial growth slows down, unlike ASF pathogen, which is inactivated at pH ranging between < 3.9 or > 11.5 [19][20].
Therefore, in order to assess disinfecting properties of the tested products or their components, it is more suitable to use different methods for different types of specific pathogens (separately for bacteria, viruses, fungi, etc.).
As part of the research work done by the Federal Centre for Animal Health Reference laboratory for ASF, the virucidal activity of commercial disinfectants against ASFV genotype II is tested. The experimental testing includes two stages: in vitro determination of the minimum effective concentration and exposure time; spraying test surfaces with working solutions of the disinfectants. Artificial organic soiling is applied at all stages to simulate near-real-life conditions.
The purpose of the experiment is to compare coded disinfectants (with the known chemical composition) based on their virucidal properties against ASF pathogen.
MATERIALS AND METHODS
Twelve disinfectants manufactured in Russia have been tested. The disinfectant samples were coded before the experiments.
Due to the absence of relevant internal regulations, the experiments were conducted according to a disinfectant test scheme similar to that given in GOST R 58151.4-2018 and R 4.2.3676-20, but after appropriate adjustment of the methods for disinfectants used for veterinary purposes and their adaptation to the current pathogen – ASFV [21].
African swine fever virus was handled and the obtained results were interpreted in accordance with the “Methodological guidelines for African swine fever virus isolation and titration in porcine spleen cell culture” [22].
Stage 1 (i.e. in vitro assessment stage) was carried out in two variants: without protein load and with the addition of inactivated bovine serum at a 40% concentration in the virus-disinfectant mixture. A 2-day subconfluent monolayer of primary porcine spleen cell culture supplemented with Eagle’s minimal essential medium, as prescribed by the Federal Centre for Animal Health, containing 10% foetal bovine serum was used as a test object.
The cell culture was inoculated with liquid-phase hemadsorbing ASFV genotype II reference strain Arm 07 deposited into the Federal Centre for Animal Health collection of strains.
Working solution of the tested disinfectants was added at a ratio of 1:9 (i.e. 1 part of virus-containing material to 9 parts of the disinfectant) to the cell debris-free suspension containing ASFV Arm 07 strain at a minimum titer of 6.0 lg HAdU50/cm3.
Seventy percent-inactivated bovine serum was used to neutralize disinfectant effect and simulate organic soiling.
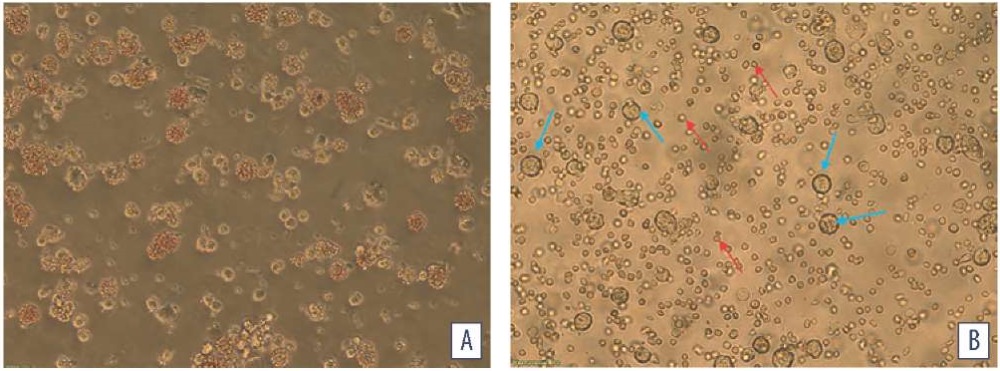
Fig. Porcine spleen cell culture: A – infected with ASFV; B – intact (blue arrows indicate porcine spleen cells, red – red blood cells)
Table 2
Results of testing virucidal activity of disinfectants against ASFV in cell culture and on a concrete test surface
|
Product code |
Components (active ingredients) |
Concentration of the active substance, % |
Exposure time, min |
Stage 1 (suspension method) |
Stage 2 (spraying) |
||
|
without protein load |
with protein load |
without protein load |
with protein load |
||||
|
1 |
potassium peroxymonosulfate |
0.55 |
30 |
+ |
+ |
+ |
+ |
|
2 |
potassium peroxymonosulfate |
0.5 |
30 |
+ |
+ |
+ |
+ |
|
3 |
potassium peroxymonosulfate |
1.5 |
60 |
+ |
+ |
+ |
+ |
|
4 |
chlorine dioxide |
0.002 |
3 |
+ |
+ |
– |
– |
|
5 |
glutaraldehyde |
0.0375 |
15 |
+ |
+ |
+ |
+ |
|
benzalkonium chloride |
0.025 |
||||||
|
6 |
alkyldimethylbenzylammonium chloride |
0.03 |
30 |
+ |
+ |
+ |
+ |
|
glutaraldehyde |
0.05 |
||||||
|
7 |
glutaraldehyde |
0.024 |
15 |
+ |
+ |
– |
– |
|
alkyldimethylbenzylammonium chloride |
0.01 |
||||||
|
8 |
didecyldimethylammonium chloride |
0.0156 |
15 |
+ |
+ |
+ |
+ |
|
alkyldimethylbenzylammonium chloride |
0.034 |
||||||
|
glutaraldehyde |
0.0214 |
||||||
|
9 |
glutaraldehyde |
2.0 |
10 |
+ |
+ |
+ |
+ |
|
alkyldimethylbenzylammonium chloride |
1.7 |
||||||
|
didecyldimethylammonium chloride |
0.8 |
||||||
|
isopropyl alcohol |
1.0 |
||||||
|
10 |
alkyldimethylbenzylammonium chloride |
0.018 |
5 |
+ |
+ |
+ |
+ |
|
glutaraldehyde |
0.015 |
||||||
|
formaldehyde |
0.01 |
||||||
|
polyhexamethylene guanidine |
0.0006 |
||||||
|
isopropyl alcohol |
0.004 |
||||||
|
11 |
sodium carbonate peroxosolvate |
0.012 |
30 60 90 |
+ + + |
+ + + |
– + + |
– + + |
|
sodium alkylbenzene sulfonate |
0.75 |
||||||
|
citric acid |
0.135 |
||||||
|
methylene blue |
0.36 |
||||||
|
sodium carbonate |
0.00015 |
||||||
|
12 |
didecyldimethylammonium chloride |
0.05 |
15 |
+ |
+ |
+ |
+ |
|
alkyldimethylbenzylammonium chloride |
0.15 |
||||||
|
glutaraldehyde |
0.05 |
||||||
|
amino oxide |
0.05 |
||||||
|
isopropyl alcohol |
0.05 |
||||||
|
isotridecanol ethoxylated |
less than 0.05 |
||||||
|
“+” – virucidal effect in place (absence of hemadsorption); “–” – no virucidal effect (presence of hemadsorption). |
|||||||
The resulting samples (both with serum and without) were kept at room temperature during the exposure time, and subsequently neutralized with bovine serum at a 1:1 ratio (1 part of sample and 1 part of the neutralizer).
Then the mixture (virus, disinfectant and neutralizer) samples were added into the plate wells with a monolayer of ASFV-sensitive porcine spleen cell culture, 30 minutes later the mixture was removed and replaced with the supportive medium. The cell culture was incubated in 5% CO2 atmosphere for 7 days at 37 °C, results monitored daily.
Stage 2 of disinfection efficacy testing included spraying 10 × 10 cm concrete test plates with working solutions from a spray bottle. Before the experiment, all surfaces were mechanically cleaned (washed with soap and brush, rinsed with running water, then wiped several times with a sterile wet cloth) and autoclaved.
The test plates were placed horizontally; ASFV suspension was pipetted onto each plate (minimum titer of 6.0 lg HAdU50/cm3) at a rate of 0.5 cm3/m2; with 5% inactivated bovine serum added to cover 100 cm2 area, and was evenly distributed over the surface. The virus-contaminated surfaces got dry at room temperature, and then were treated with the tested disinfectant solution at the minimum effective concentration and exposure time.
To simulate organic contamination (protein load), 40%-inactivated bovine serum was used, which was applied to virus-contaminated surfaces. Surfaces were then sprayed with the test product at the application rate of 0.3 L/m² (per manufacturer’s protocol).
Control test plates were sprayed with sterile or boiled tap water at the same application rate (0.3 L/m²) as in the experimental procedure. In order to determine completeness of ASFV inactivation, swabs were sampled from the test surfaces and then applied onto a sensitive porcine spleen cell culture (virus detection was performed through virus isolation with three blind passages conducted in cell culture for each sample).
Results were examined by the presence or absence of hemadsorption phenomenon – a qualitative specific indicator of ASFV replication. The disinfectant sample was considered to exhibit virucidal activity, if no hemadsorption was observed.
RESULTS AND DISCUSSION
Table 2 provides results of disinfectant efficacy testing (judged by the presence or absence of virucidal activity) for coded disinfectant samples during two consecutive in vitro stages.
It was found that nine out of twelve disinfectants were able to inactivate highly virulent ASFV reference strain Arm 07 at all concentrations and exposure times, prescribed by manufacturers, on sprayed concrete test surfaces.
Disinfectants coded as 1, 2 and 3 with potassium peroxymonosulfate as the main active ingredient (at concentrations of 0.55, 0.5 and 1.5%, respectively) exhibited activity against ASFV both when tested by suspension method and by spraying onto the concrete test surfaces, during 30 and 60 minute-exposure times. Potassium peroxymonosulfate is able to inactivate ASFV even with significant organic contamination, but in this case its concentration should be at least 1.0% [23]. The research findings demonstrate efficacy of potassium peroxymonosulfate-based disinfectants even at lower concentration (both with and without protein load), which can be taken into account when optimizing chemical composition of disinfectants.
The disinfectant coded as 4 (0.002% chlorine dioxide working solution) demonstrated sufficient virucidal efficacy at experiment stage 1, however, ASFV persisted on the concrete test surface after 3-minute exposure. The tested concentration of ClO2 exceeds the recommended one (0.0012%) for inactivation of ASF pathogen; and the lack of activity is most likely caused by insufficient exposure time. To achieve optimal virus degradation efficacy, when applying chlorine dioxide to the surface, it is necessary to adhere to a strict time-temperature regimen (at least 50 min, 37 °C) [24].
Disinfectants coded as 5, 6, 7, 8, 9, 10 and 12 contain glutaraldehyde in their composition. Disinfectant 7 did not show pronounced virucidal activity in the experiment, despite sufficiently high concentration of the active substance (0.024%) in the working solution (as declared by the manufacturer) exceeding the efficacy rates of other disinfectants, for example, the one coded as 10 and containing only 0.015% glutaraldehyde, but proved effective against ASFV during the experiment. The available data from the few existing studies show the possibility to use glutaraldehyde at 0.1% concentration during 30-minute exposure [23]. The lack of efficacy of disinfectant No. 7 may be explained either by insufficient levels of excipients in its formulation or deviations in its manufacturing process. The critical lack of data on the minimum effective concentration of glutaraldehyde required for ASFV inactivation highlights the need for research to assess the virucidal efficacy of the disinfectants. It should be noted that glutaraldehyde concentration of more than 0.5% can increase cytotoxicity, which significantly limits the range of experiments [25].
Disinfectant No. 11, containing 0.012% sodium carbonate peroxosolvate, 0.75% sodium alkylbenzene sulfonate, 0.135% citric acid, 0.36% methylene blue, 0.00015% sodium carbonate, showed partial virucidal efficacy against the pathogen. The disinfectant exhibited no virucidial efficacy against ASFV during 30-minute exposure at the declared concentrations, however, with prolonged exposure time, namely 60 and 90 minutes, it was quite effective. The individual components in its composition, according published data, demonstrate activity against ASF pathogen at high concentrations and during prolonged exposure time [26]. Chlorine compounds are widely used as disinfectants due to their high efficacy resulting from the ability to denature proteins; however, they have a corrosive effect and are inhibited by organic substances. Sodium alkylbenzene sulfonate is classified as an anionic surfactant with well-pronounced detergent properties. The virucidal properties of citric acid were confirmed when used at 2% concentration during 30-minute exposure; the effect is achieved due to interaction of lipophilic structures with the virus membrane and decreased pH. Sodium carbonate also proved effective against ASFV at 1% concentration and after 30-minute exposure [26].
The conducted tests demonstrate that protein load (as a simulation of organic contamination), short exposure time, and relatively low temperatures (of the working solution, treated object, and environment) are all critical factors that can significantly reduce efficacy of disinfectants. Therefore, thorough mechanical pre-cleaning of surfaces and strict adherence to recommendations, including those on exposure time and temperature regime, is still of great importance for practice, which agrees with published literature [17].
CONCLUSION
During experiment stage 1 (in porcine spleen cell culture) all the 12 tested disinfectants exhibited virucidal activity against ASFV reference strain Arm 07, which confirms in vitro efficacy and absence of cytotoxic effects, if the recommended concentrations and exposure time are complied with.
During experiment stage 2 (on concrete test surfaces with or without simulation of organic contamination), only 9 out of 12 disinfectants were effective, which underscores the need for comprehensive studies to assess the virucidal activity of disinfectants using various test objects.
To optimize composition of disinfectants in order to increase their efficacy against ASF pathogen, we consider it advisable to use glutaraldehyde at a minimum concentration of 0.05%, potassium peroxymonosulfate at a minimum concentration of 0.5%.
The experimentally confirmed impact of organic contaminants on disinfectant virucidal efficacy shows that for practical purposes it is extremely important to thoroughly clean surfaces and select the most effective products for both disinfection and washing. In addition, it is necessary to account for other factors, including a composition and concentration of active ingredients that are effective against the specific pathogen, ambient air temperature, temperature of treated surfaces, temperature of the disinfectant working solution, exposure time, drying parameters and other relevant variables.
Application of those commercial disinfectants with the unknown virucidal activity against ASF pathogen increases the risk of undermining substantial efforts invested in preventive and eradication measures. Therefore, there is a need to introduce relevant regulatory framework on testing commercial disinfectants for their efficacy against currently circulating pathogens of infectious diseases listed in the instruction for use.
References
1. Petrova O. N., Karaulov A. K. Sravnitel’nyi analiz ushcherba ot rasprostraneniya osobo opasnykh boleznei zhivotnykh na territorii RF za 2018–2020 gg. s elementami prognoza na 2021 g. = Comparative analysis of the damage caused by spread of highly dangerous animal diseases in the Russian Federation for 2018–2020, with projections for 2021. BIO. 2021; (5): 12–18. https://elibrary.ru/pwuhnj (in Russ.)
2. WOAH. Terrestrial Animal Health Code. https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access
3. WOAH. Manual of Diagnostic Test and Vaccines for Terrestrial Animals. https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access
4. Veterinary rules for implementation of preventive, diagnostic, restrictive and other measures, imposing and lifting quarantine and other restrictions to prevent spread and eradicate outbreaks of African swine fever: approved by Order of the Ministry of Agriculture of Russia No. 37. 28.01.2021. https://docs.cntd.ru/document/573473462?ysclid=m6abw80wls994110372 (in Russ.)
5. Makarov V. V., Sukharev O. I., Litvinov O. B. African swine fever virus: resistance, persistence, decontamination. Veterinariya. 2012; (9): 23–25. https://elibrary.ru/pdslxt (in Russ.)
6. Makarov V. V., Grubyy V. A. African swine fever: epizootic polymorphism and control. Part II. History and geography of successful eradication. Veterinary Science Today. 2013; (3): 21–25. https://elibrary.ru/ratovp (in Russ.)
7. Sauter-Louis C., Conraths F. J., Probst C., Blohm U., Schulz K., Sehl J., et al. African swine fever in wild boar in Europe – a review. Viruses. 2021; 13 (9):1717. https://doi.org/10.3390/v13091717
8. Vlasov M. E., Slivko I. A., Sereda A. D. Persistence of African swine fever virus in environmental objects. Veterinariya. 2018; (10): 17–22. https://doi.org/10.30896/0042-4846.2018.21.10.17-22 (in Russ.)
9. Beltran-Alcrudo D., Arias M., Gallardo C., Kramer S. A., Penrith M. L., Kamata A., Penrith M. L. African Swine Fever: Detection and Diagnosis – a manual for veterinarians. Rome: FAO; 2017. 88 p. https://openknowledge.fao.org/handle/20.500.14283/i7228en
10. Vlasov M. E. Biological properties of African swine fever virus isolates recovered in various regions of the Russian Federation from wild boars and domestic pigs: Author’s thesis for degree of Cand. Sci. (Veterinary Medicine). Volginsky; 2021. 149 p. (in Russ.)
11. Sindryakova I. P., Morgunov Yu. P., Chichikin A. Yu., Gazaev I. Kh., Kudryashov D. A., Tsybanov S. Zh. The influence of temperature on the Russian isolate of African swine fever virus in pork products and feed with extrapolation to natural conditions. Agricultural Biology. 2016; 51 (4): 467–474. https://doi.org/10.15389/agrobiology.2016.4.467eng
12. SanPiN 3.3686-21 Sanitary and epidemiological requirements for prevention of infectious diseases: approved by resolution No. 4 of the Chief State Sanitary Doctor of the Russian Federation 28.01.2021 (as amended on 25.05.2022). https://docs.cntd.ru/document/573660140?ysclid=m6akzus7b3685301845 (in Russ.)
13. Rules for disinfection and decontamination at the facilities under state veterinary surveillance: approved by Ministry of Agriculture of Russia No. 13-5-2/0525 15.07.2002. https://base.garant.ru/70268920/?ysclid=m6aleudxu5550644985 (in Russ.)
14. Botalova D. P., Kuzmin V. A., Igolkin A. S., Tsiple S. Yu., Kasatkin A. S. Viricidal activity of disinfectants “Dezon Vetklin” and “Dezon Vet” against the African swine fever virus. International Bulletin of Veterinary Medicine. 2022; (4): 16–23. https://doi.org/10.52419/ISSN2072-2419.2022.4.16 (in Russ.)
15. Gerasimov V. N., Kuzmin V. A., Vasinsky R. G. Modern disinfectants in system of measures to prevent the introduction and spread of African swine fever virus in the Russian Federation. Veterinaria Kubani. 2017; (1): 15–16. https://elibrary.ru/zduibr (in Russ.)
16. Balyshev V. M., Balysheva V. I., Bolgova M. V., Zhivoderov S. P., Selyaninov Y. O. Inactivating effect aerosols of some disinfectants on African swine fever virus. Veterinariya. 2016; (11): 44–47. https://elibrary.ru/wzixpb (in Russ.)
17. MU 3.5.2431-08 Studying and evaluating virucidal activity of disinfectant products: guidelines. Moscow: Federal Center of Hygiene and Epidemiology of the Federal Office for Inspectorate in the Field of Customers and Human Well-Being Protection; 2010. 39 p. (in Russ.)
18. Shestopalov N. V., Panteleyeva L. G., Sokolova N. F., Abramova I. M., Lukichev S. P. Federal clinical guidelines on selection of chemical disinfectant products and sterilization products to be used in medical organizations. Moscow: Remedium Privolzh’e; 2015. 56 p. https://elibrary.ru/tytfur (in Russ.)
19. Vorobiev A. A., Krivoshein Yu. S., Shirobokov V. P. Medical and sanitary microbiology: a textbook. Moscow: Academia; 2003. 464 р. (in Russ.)
20. Igolkin А. S., Gruzdev K. N. African swine fever: study guide. Vladimir: Federal Centre for Animal Health; 2021. 126 p. (in Russ.)
21. Laboratory test methods for medical and preventive disinfectants to assess their effectiveness and safety: guidelines. Moscow: Federal Center of Hygiene and Epidemiology of the Federal Office for Inspectorate in the Field of Customers and Human Well-Being Protection; 2010. 615 p. (in Russ.)
22. Mazloum A., Sharypova D. V., Gavrilova V. L., Puzankova O. S., Zhukov I. Yu., Aronova E. V., et al. Methodical guidelines for the isolation and titration of African swine fever virus in porcine spleen cell culture: approved by the Federal Centre for Animal Health on 15.03.2019 No. 09-19. Vladimir; 2019. 24 p. (in Russ.)
23. Juszkiewicz M., Walczak M., Mazur-Panasiuk N., Woźniakowski G. Virucidal effect of chosen disinfectants against African swine fever virus (ASFV) – preliminary studies. Polish Journal of Veterinary Sciences. 2019; 22 (4): 777–780. https://doi.org/10.24425/pjvs.2019.131407
24. Wei R., Wang X., Liu X., Guo C. Chlorine dioxide inhibits African swine fever virus by blocking viral attachment and destroying viral nucleic acids and proteins. Frontiers in Veterinary Science. 2022; 9:844058. https://doi.org/10.3389/fvets.2022.844058
25. Juszkiewicz M., Walczak M., Mazur-Panasiuk N., Woźniakowski G. Effectiveness of chemical compounds used against African swine fever virus in commercial available disinfectants. Pathogens. 2020; 9 (11):878. https://doi.org/10.3390/pathogens9110878
26. Beato M. S., D’Errico F., Iscaro C., Petrini S., Giammarioli M., Feliziani F. Disinfectants against African swine fever: an updated review. Viruses. 2022; 14 (7):1384. https://doi.org/10.3390/v14071384
About the Authors
I. A. LavrentievRussian Federation
Ivan A. Lavrentiev, Leading Veterinarian, Reference Laboratory for African Swine Fever,
Yur’evets, Vladimir 600901.
A. S. Igolkin
Russian Federation
Alexey S. Igolkin, Cand. Sci. (Veterinary Medicine), Head of Reference Laboratory for African Swine Fever,
Yur’evets, Vladimir 600901.
A. А. Shevtsov
Russian Federation
Alexander А. Shevtsov, Cand. Sci. (Veterinary Medicine), Leading Researcher, Information and Analysis Centre,
Yur’evets, Vladimir 600901.
I. S. Kolbin
Russian Federation
Ivan S. Kolbin, Junior Researcher, Reference Laboratory for African Swine Fever,
Yur’evets, Vladimir 600901.
O. S. Puzankova
Russian Federation
Olga S. Puzankova, Cand. Sci. (Veterinary Medicine), Senior Researcher, Reference Laboratory for African Swine Fever,
Yur’evets, Vladimir 600901.
Vera L. Gavrilova
Russian Federation
Vera L. Gavrilova, Cand. Sci. (Biology), Researcher, Reference Laboratory for African Swine Fever,
Yur’evets, Vladimir 600901.
R. S. Chernyshev
Russian Federation
Roman S. Chernyshev, Junior Researcher, Reference Laboratory for African Swine Fever,
Yur’evets, Vladimir 600901.
Review
For citations:
Lavrentiev I.A., Igolkin A.S., Shevtsov A.А., Kolbin I.S., Puzankova O.S., Gavrilova V.L., Chernyshev R.S. Virucidal activity of disinfectants against African swine fever virus. Veterinary Science Today. 2025;14(2):156-163. https://doi.org/10.29326/2304-196X-2025-14-2-156-163



































