Scroll to:
Metal nanoparticles, silver nanoparticles and their impact on human and animal health (review)
https://doi.org/10.29326/2304-196X-2025-14-1-90-100
Abstract
Introduction. Due to increased prevalence of different diseases and antimicrobial resistance development in recent year, such advancements of the humankind as nanomaterials have gained the significance. A relatively small amount of data (lack of data) on biological distribution, pharmacokinetics and potential toxicity of nanometals for the organism hinders the development of safer and more effective drugs.
Objective. Analysis and summary of data published in modern scientific literature on studies of metal nanoparticles and silver nanoparticles, their distribution and impact on human and animal health, as well as their use in biomedicine and veterinary medicine.
Materials and methods. Publications were searched for in eLIBRARY.RU, cyberleninka.ru, scholar.google.ru, www.mdpi.com, www.researchgate.net, www.sciencedirect.com, PubMed database. The literature published during last six years and more recent publications have been used.
Results. Nanostructures can be organic, inorganic and hybrid. One of the most studied inorganic materials are metal nanoparticles. They are widely used both in engineering and biomedicine, in particular in veterinary medicine, as bactericidal and virucidal agents, anti-cancer drugs and diagnostic tools. In the CIS members, silver nanoparticles are most commonly used. It is known that shape, size and surface electric charge affect the antibacterial activity of nanostructures. Several types of silver-based drugs are available at the market now: colloidal, silver cluster and zerovalent silver. Zerovalent silver-based drugs are least toxic. Nanoparticle-based drugs can reach target tissues through local administration such as oral, inhalation, subcutaneous administration, and directly into blood flow by intraperitoneal or intravenous injection. Biodistribution of metal nanostructures depends on particle type, their size, surface, interaction with proteins as well as routes of exposure, doses and hydrophobic properties. Pharmacokinetics of silver nanoparticles does not differ from that of metal nanoparticles, furthermore nanosilver does not accumulate in spleen, liver, kidneys and lungs which is potentially toxic.
Conclusions. Further in-depth studies of nanoparticle biodistribution, compatibility and potential toxicity are needed to facilitate the development of more effective and safe therapeutic drugs.
Keywords
For citations:
Sumarokova A.D., Statsevich L.N. Metal nanoparticles, silver nanoparticles and their impact on human and animal health (review). Veterinary Science Today. 2025;14(1):90-100. https://doi.org/10.29326/2304-196X-2025-14-1-90-100
INTRODUCTION
Overuse of antimicrobials inhibits the symbiotic microflora and contributes to the development of drug-resistant pathogens, thus hindering the drug therapeutic action and causing side effects and complications. New resistant mechanisms of some pathogens threaten the scope of treatment for many infectious diseases. Successful treatment of even common illnesses such as pneumonia, sepsis, and foodborne diseases is hindered and sometimes impossible due to the reduced effectiveness of antimicrobials1. The growing global antimicrobial resistance (AMR) concern has created an urgent need to reduce the use of antimicrobials and search for the most effective drugs to replace them [1].
The field of nanotechnology is growing rapidly. The use of nanomaterials to face biomedical and veterinary challenges, such as the diagnosis and treatment of various diseases, is currently one of the priority scientific trends. Metal nanoparticles (MNPs) proposed for use in public and animal health possessing unique chemical and biological properties that make them versatile in their functions are of particular interest among the wide range of nanoparticles (NPs) [2].
Currently, there are three main groups of action of nanostructures on biological objects:
1) modification (iron and copper NPs);
2) toxicity (NPs of copper, aluminum oxide, silver, iron, iron hydroxide);
3) mutagenicity (NPs of silicon, nickel hydroxide, iron oxide, titanium dioxide, gold, zinc oxide, copper oxide and silver) [2].
The most commonly used metal NPs are silver, gold, iron oxide, copper and zinc [3]. In veterinary medicine, these nanomaterials are mainly used as antiviral and antimicrobial agents [4].
Silver nanoparticles (AgNPs) are of particular interest among MNPs. They are mainly used for antimicrobial and anticancer therapy. AgNPs are also applied in the promotion of wound repair and bone healing, or as the vaccine adjuvants and anti-diabetic agents [5].
While nanotechnology is regarded as one of the foremost technologies already applied in diverse subjects, its application in veterinary science is still in its infancy stages when compared to other sister disciplines. Herewith, it already has revealed new opportunities in molecular biology, biotechnology, and has revolutionized virtually all veterinary medicine and animal science disciplines these days by offering new, small-scale devices and materials that are beneficial to living organisms [6]. NPs increasingly invade animal therapeutics, diagnostics, production of veterinary vaccines, used as farm disinfectants, for animal breeding and reproduction, and even in the field of animal nutrition. Their replacement of commonly used antibiotics directly reflects on the public health, as they minimize the problem of drug resistance in both human and veterinary medicine, and the problem of drug residues in milk and meat [7].
Nanometals-based products, particularly AgNP-based products, are actively studied and used as antimicrobial, antiviral, antifungal [8] and antitumor agents, as well as analgesic drugs [9] and dietary supplements to increase animal performance, improve their immunity, and even as a part of a synergetic anti-AMR bacteria system [10][11][12][13].
Despite the fact that nanoobjects are already being used to solve various biomedical and veterinary problems, there is currently insufficient data on the bio-distribution of nanoelements in the body. At the same time, understanding the patterns of NPs distribution in the body, taking into account their different composition and structure, is of paramount importance for identification of the prospects of their further biological and medical use [14].
The paper reviews the achievements in nanomaterial use over the past 20 years. This review is intended to provide valuable information for researchers interested in the medical and veterinary applications of MNPs, namely of AgNPs.
The purpose of the work is to analyze and summarize the data of modern scientific literature on the study of MNPs and AgNPs, in particular in the field of biomedicine and veterinary medicine, as well as on the study of their distribution and impacts on the human and animal body.
MATERIALS AND METHODS
The research data concerning the study of MNPs, namely of AgNPs, over the past 20 years have been used for this review. The published data on the properties of MNPs and AgNPs, their impact on the human and animal body, and their application in the field of veterinary medicine and biomedicine have been analyzed.
The literature was searched and analyzed using the following online resources: eLIBRARY.RU, cyberleninka.ru, scholar.google.ru, www.mdpi.com, www.researchgate.net, www.sciencedirect.com, PubMed databases.
Foreign and domestic reviews (57%) on NPs, their types, synthesis, distribution and impacts on the body, application in various fields; and research papers (43%), presenting the results of the studies on the use of MNPs and AgNPs as diagnostic agents, therapeutic drugs, dietary supplements and others have been selected for the analysis. 66% of the publications analyzed have been published over the past 6 years (11.5% of them were published in 2023, 7.7% in 2024), and 34% are earlier studies.
TYPES OF NANOPARTICLES USED IN MEDICINE AND DIAGNOSTICS
To date, many types of NPs have been developed that are used in the biomedical and veterinary fields. NPs are divided into organic, inorganic and hybrid. Most organic nanostructures are biocompatible, biodegradable, and non-toxic, while most inorganic NPs are smaller in size, exhibit better penetration capability, drug loading capacity, excellent stability, tunable degradation rates and release profile [15][16][17][18][19].
Inorganic NPs include particles of metals or their oxides, semiconductor NPs (silicon oxide), which include quantum dots, as well as carbon derivatives (graphene, fullerenes, carbon nanotubes). Organic nanoobjects are represented by structures based on lipids and their derivatives (liposomes, lipid NPs, micelles), as well as synthetic compounds of a polymeric nature: linear (classical) and branched (dendrimers, dendrons) [15].
In this review, inorganic NPs, namely MNPs, are focused on. They can be produced in the form of spheres, nanocapsules, rods and other shapes that are highly stable and effective in various conditions with easily controlled physicochemical properties. Unfortunately, MNPs have some drawback: the complexity of manufacturing (uniformly sized, homogeneous in shape and surface charge) and the difficulty of eliminating them from the body [20].
The most commonly used MNPs in biomedicine are gold, silver, copper oxide, zinc oxide, magnesium oxide, iron oxide, titanium dioxide, and aluminum NPs [21][22][23][24][25].
Silver nanoparticles have long been widely studied to be used in various fields of biomedicine and veterinary medicine due to their antimicrobial properties and antioxidant activity [26]. They are effective both against gram-negative and gram-positive bacteria and are incorporated into fabric wound dressings [27].
Gold nanoparticles are another group of MNPs that are being extensively explored and have shown promise in medicine and diagnostics, for example, antibacterial, in anticancer therapy for targeted drug delivery and reducing the tumor growth. In addition, AgNPs are used in spectroscopy and to enhance optical imaging [28][29].
In addition, the last frequently explored group of MNPs are metal oxides. The use of zinc oxide, ZnO; copper (II) oxide, CuO; magnesium oxide, MgO; titanium (IV) oxide, TiO2; aluminum oxide, Al2O3; iron (II, III) oxide, Fe3O4 has been studied for a long time [30][31][32][33][34][35][36][37].
Iron oxide is currently gaining popularity due to its magnetic properties. Iron oxide NPs are used as drug delivery vehicles, for magnetic resonance imaging, cancer diagnosis, and tissue engineering [30]. Tin oxide has unique electrical properties that depend on the size of its NPs [31]. Tungsten trioxide is often used as sensing material for chemiresistive gas sensors [32]. Titanium dioxide conducts electricity, therefore, it has found application in optical and solar energy, as well as in the medical, food and microbiological industries for the photocatalytic sterilization [33][34]. Magnesium oxide NPs are utilized to reduce air pollution and as catalysts for organic reactions [35]. Copper oxide NPs have found applications in various catalytic fields, including oxidation and photothermy [36]. Magnesium, copper, aluminum, and zinc oxides have also proven to be potential antibacterial and antifungal agents [37].
SCIENTIFIC INTEREST IN SILVER AND GOLD NANOPARTICLES AND THEIR ANTIBACTERIAL ACTIVITY
Over the past 20 years, the number of publications found for “gold nanoparticles in medicine” search query has changed annually on Google Scholar2 web search engine from 1,360 links in 2003 to 61,900 in 2023, with the largest number found in 2022 (67,800). For “silver nanoparticles in medicine” search query there were 904 publications found in 2003, and in 2023, their number reached 51,500; the largest number (56,600) was also available in 2022 (Fig. 1).
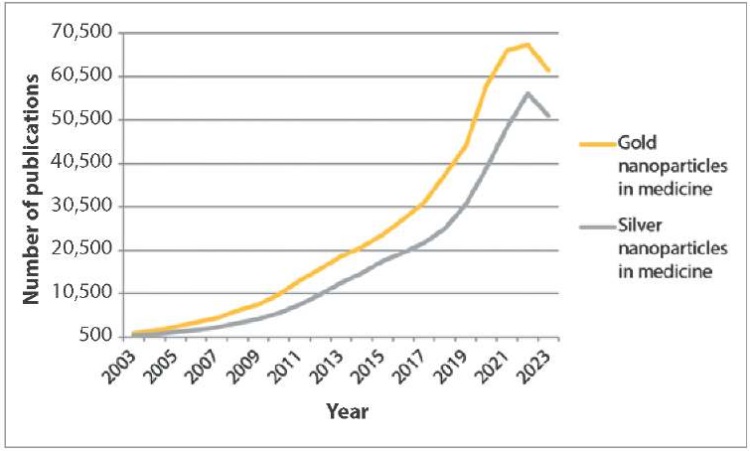
Fig. 1. Publications in English for “gold nanoparticles in medicine” and “silver nanoparticles in medicine” search queries in Google Scholar from 2003 to 2023
The number of publications for “gold nanoparticles in veterinary medicine” and “silver nanoparticles in veterinary medicine” search queries is almost 9 times less (Fig. 2). The lowest number of links in English on these topics was observed in 2003 (127 and 103, respectively). The largest number of publications for “gold nanoparticles in veterinary medicine” query was available in 2023 (7,570). The number of links for “silver nanoparticles in veterinary medicine” in the same year was the largest in the last 20 years (7,910).
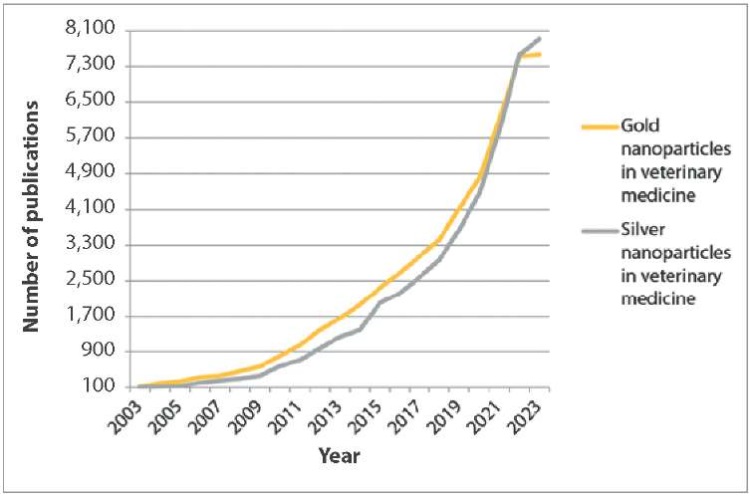
Fig. 2. Publications in English for “gold nanoparticles in veterinary medicine” and “silver nanoparticles in veterinary medicine” search queries in Google Scholar from 2003 to 2023
For “наночастицы золота в медицине” search query in Russian, there were only 12 publications on the same web search engine in 2003 and 225 in 2023 (Fig. 3). For “наночастицы серебра в медицине” search query in Russian, 395 publications were found in 2023, whereas in 2003 there were only 20. The largest number of publications on “наночастицы золота в медицине” was in 2018 (438), and on “наночастицы серебра в медицине” – in 2016 (636).
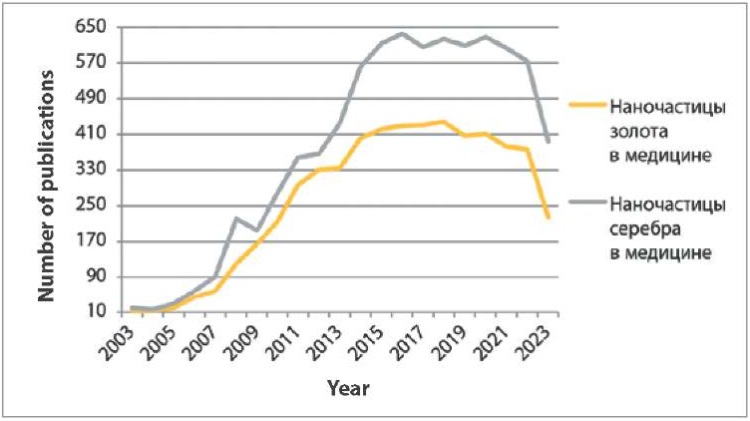
Fig. 3. Publications in Russian for “наночастицы золота в медицине” and “наночастицы серебра в медицине” search queries in Google Scholar from 2003 to 2023
The number of publications on “наночастицы золота в ветеринарии” and “наночастицы серебра в ветеринарии” in Russian was 5–8 times less than the search results for NPs in the medicine. At the same time, in 2005 absolutely no links were found for each of the search queries (Fig. 4).
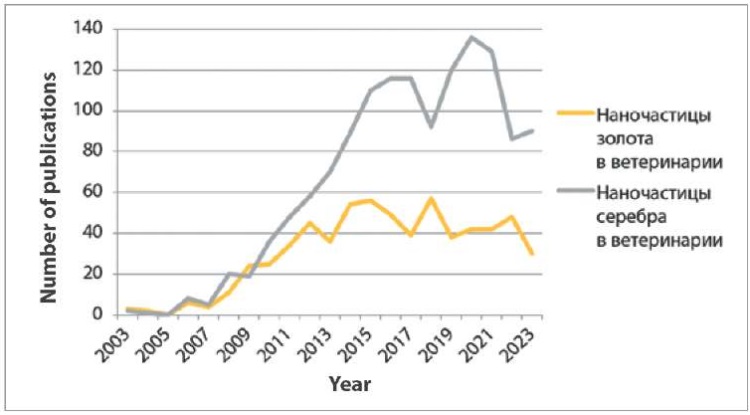
Fig. 4. Publications in Russian for “наночастицы золота в ветеринарии” and “наночастицы серебра в ветеринарии” search queries in Google Scholar from 2003 to 2023
The largest number of publications on “наночастицы серебра в ветеринарии” was in 2020 (136), and on “наночастицы золота в ветеринарии” – in 2018 (57).
Further analyzing the interest in the use of nanostructures in medicine, 569 queries on “наночастицы золота в медицине” were recorded in the Yandex search engine from January 2018 to December 2023 (Fig. 5). The largest number of queries was in 2022 (140). The number of queries on “наночастицы серебра в медицине” on yandex.ru3 during the same period was 749. The peak of popularity of search queries on this topic occurred in 2023 (202 requests).
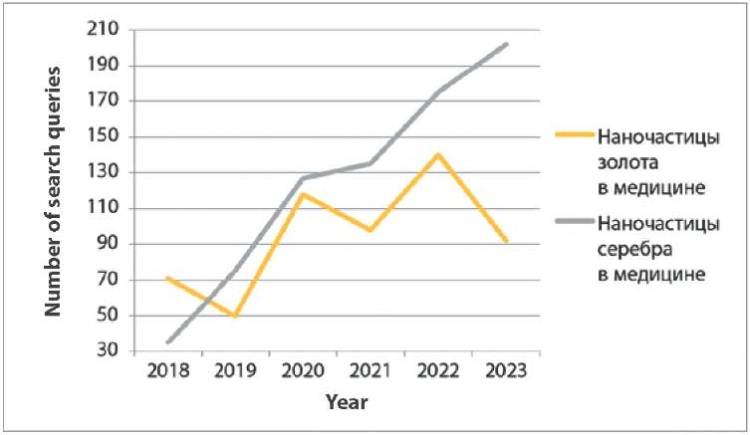
Fig. 5. Publications for “наночастицы золота в медицине” and “наночастицы серебра в медицине” search queries in Yandex from 2003 to 2023
At the same time, there have been no search queries for “наночастицы золота в ветеринарии” and “наночастицы серебра в ветеринарии” on this engine over the past 5 years.
The graphs show (Fig. 1–4) that links in English on nanostructures in medicine and veterinary medicine exceed the number of links in Russian by more than 100 times. Also, based on the number of publications appearing during the year, it is clear that foreign colleagues’ interest in MNPs in medicine decreased only a year ago, in contrast to the interest of our colleagues, which has tended to decrease over the past five years. At the same time, data from Yandex Wordstat suggest an annual increase in the interest of scientists from Russia and the CIS countries in AgNPs in the field of biomedicine. Gold nanoparticles have not aroused stable interest among researchers over the past six years (Fig. 5).
It has been established that the number of foreign studies in the field of veterinary medicine devoted to MNPs is increasing every year (Fig. 2). Russian-speaking veterinary scientists were not consistently interested in investigation of gold and silver NPs since 2015–2016 (Fig. 4).
A difference of 8–9 times between the number of links, both in English and in Russian, on the use of NPs in medicine and in veterinary medicine may be due to the fact that NPs in veterinary practice have not yet found such wide application as in public health. However, research on the use of NPs to treat and diagnose diseases of pets and livestock is also conducted. Vaccines are being developed against a number of significant bacterial and viral diseases, such as equine influenza, bovine viral diarrhea, and Newcastle disease, as well as NPs-based carriers for the delivery of imaging tools, antibiotics, vitamins, and drugs, including those targeting neoplastic diseases [38].
Thus, despite the declining interest of Russian scientists in investigation of nanomaterials use in medicine and veterinary medicine, judging by the number of publications on this topic, our compatriots are more interested in AgNPs and their use as a base for therapeutic drugs. And this is not surprising, because NPs have long been the most widely used antibacterial nanoagents due to their broad spectrum of action against a variety of bacteria, viruses and fungi [39].
The earliest known reference to the use of silver in medicine goes back to the 19th century, when it was used to prevent gonococcal conjunctivitis in newborns, and later, in the 20th century, silver was used by surgeons for local treatment of burn wounds and as internal antiseptics [40][41][42].
Colloidal silver has been produced in a wide variety of forms for over 100 years. Currently, there are many ways to synthesize more effective forms of colloidal AgNPs.
The methods of synthesis of nanoparticles can be conditionally divided into two groups: reduction of silver ions (Ag+) and dispersal to nanoscale sizes. The first group involves chemical methods, and the second group involves physical methods. At the same time, nanoscale silver can have various geometric shapes: spherical, pyramids, rods, cubes, etc. [43]. The bactericidal effect of NPs depends on different parameters including size, shape, and the surface charge of the particles [39].
The shape of nanoparticles. As shown by the results of studies conducted in 2016 and 2019 aimed to explore the effect of NPs shapes and facets on antibacterial activity, crystalline particles with a high-atom-density and a higher number of facets have better activity against bacteria. For example, triangular silver nanoprisms with 111 facets have a higher atom density and, accordingly, exhibited better antibacterial efficiency than that of the spherical and rod-shaped silver particles with 100 and 110 facets [44, 45]. For example, S. Pal et al. synthesized spherical, rod-shaped NPs and truncated triangular nanoplates, and then evaluated their antibacterial activities against E. coli in solution and on agar plates. The researchers concluded that truncated triangular nanosilver exhibited the highest biocidal activity followed by silver nanospheres and nanorods. Scanning transmission electron microscopy revealed that all nanostructures can bind to membrane surface, alter the cell membrane permeability and subsequently cause the cell death. However, truncated nano-triangles present the highest percentage of exposed facets, which favor the direct interaction with the main components of the cell membrane, lead to the enhanced surface binding, cell uptake, and efficiently killing of bacteria [39].
Helmlinger J. et al. studied the effect of NPs shape on Staphylococcus aureus. They concluded that nanoplatelets exhibited the highest toxicity, followed by nanospheres, nanorods, and finally nanocubes [46].
The size of the nanoparticles. Experimental studies observed that the antibacterial activity was directly proportional to the decrease in NPs size: efficiency decreased as NP size increased. For example, AgNPs with a size of 1 to 10 nm are more effective in inhibiting bacterial growth [46][47][48]. This is probably due to the concentrated accumulation of NPs in the cell membrane and cytoplasma of microorganisms [49][50]. It is also suggested that the increased antibacterial activity may be because of the fact that smaller nanoelements are able to release their toxic components at a higher rate due to higher surface-to-volume ratio as the size of the NPs decreases [47][51]. In addition, recent studies showed that small and medium sized AgNPs strongly affect mitochondrial electron transport, autophagy and phagocytosis, and the integrity and organization of organelles [52].
The surface charge of nanoparticles. The antimicrobial activity of NPs can be changed by modifying their surface charge. Hu C. et al. demonstrated that AgNPs with a positive surface charge have increased antibacterial activity [53]. Antimicrobial activity is also mediated by released Ag+ ions from NPs surface. This occurs because of oxidative dissolution: first, metallic silver is oxidized in the presence of dissolved oxygen, and then the formed basic oxide is dissolved in acidic conditions. Silver ions also possess high affinity to electron-donating groups that are extensively present on membrane or proteins. Ag+ ions can readily coordinate with DNA, RNA, peptides forming the insoluble compounds and thus hindering the cell division and reproduction [39].
SILVER NANOPARTICLE-BASED DRUGS
It can be said that rapidly developing AMR brings silver-based drugs to the forefront again.
Currently, there are several types of silver-based drugs of different dosage forms available on the pharmaceutical market.
The most well known drugs are based on colloidal (cationic) silver (Ag+): these are silver oxide, silver salts (nitrates, sulfates, phosphates), silver complexes (citrates or lactates), and free silver aqueous cations. Colloidal silver products available on the market are “Tinosan SDC” (BASF, Germany), “Argolife” (Art Life, Russia), silver sulfate (Aurat, Russia) [54].
There are also metallic micro-dispersed and nano-dispersed forms of silver products (cluster silver), in which the main amount of silver is in the low-toxic metallic form Ag0. Cluster silver products are highly effective and less toxic than products containing a higher amount of cationic silver [55]. Such products include: “AgBion-2” (Concern “Nanoindustria”, Russia), “Argovit” (Vector-Vita, Russia), “Poviargol” (Institute of macromolecular compounds, Russia), “Argonica” (VectorPro, Russia).
Nulvalent (metallic) silver is a separate category of products, namely colloidal ion-free silver (Ag0), for example, of the trademark “KND” (Sentosa Factoring NP, Russia): colloidal silver concentrate “KND-S”, colloidal silver and copper concentrate “KND-SM”, colloidal silver concentrate “KND-S-K”, cosmetic raw materials and supplements “AREGONA (KND-SP)” [54].
As noted above, preparations containing silver in a finely dispersed form are significantly less toxic than products based on silver salts. Nulvalent silver products are much less toxic than those based on cluster silver. This is due to the almost complete absence of cationic Ag in nulvalent silver.
Cationic silver is also reduced in its composition and incompatible with many components of practical systems (for example, with saline solutions), unlike cluster and nulvalent silver, which are more compatible and stable [55].
Nanosilver-based drugs are very promising for use in veterinary medicine and zootechnics. AgNPs can be used for biosafety purposes on farms, for hatchery fumigation, sterilization of poultry houses and cages. It has been found that AgNPs can improve the adaptive immune system of birds [56] and hatching rates [57]. In 2023, physiologically stable, bio-compatible AgNPs were produced which may be used for targeted drug delivery in veterinary medicine that could offer enhanced therapeutic efficacy with minimal side effects [58]. In the same year, it was found that the addition of nanosilver to milk fed to calves has a positive effect on their metabolism. Therefore, nanosilver can be used to prevent infectious diseases of calves during the first month after their birth, which will mitigate the risks of AMR development and improve livestock production performance [59].
DISTRIBUTION OF NANOPARTICLES IN THE BODY
The distribution of a pharmaceutical substance containing nanoelements in organs and tissues changes significantly, affecting the pharmacodynamics of the drug. In this regard, the study of NPs biodistribution is the most important stage in the studies [60]. However, currently most NPs are still in the preclinical evaluation phase with few approved for clinical use. Most articles are devoted to in vitro studies of nanomaterials and there are relatively few publications on in vivo biodistribution studies. At the same time, the lack of concrete data on the distribution and accumulation of NPs in organs and tissues limits their application [61][62].
Pharmacokinetic studies are needed to assess the distribution of NPs and their toxicity. Absorption, distribution, metabolism and elimination are the four processes that make up pharmacokinetics [63]. Few pharmacokinetic studies of nanoforms have been conducted, and only nanomaterials are controlled4,5, but there are no standards and regulations regarding NPs biodistribution, which makes the evaluation of this parameter difficult.
The biodistribution of MNPs depends on NPs’ type, size, surface charge, protein binding, effects, dose and/or hydrophobicity [62][63].
The rate and degree of absorption are influenced by the physiological environment and the NPs’ characteristics. Nanoformulations pass across physiological and physical barriers that selectively block the transport of molecules, reducing NPs bioavailability. Cellular uptake is heavily influenced by size, surface charge, and shape [64][65]. The route of administration and the characteristics of the NPs affect absorption [62].
The MNPs with a negative surface charge has a higher absorption rate at the gastrointestinal membrane in the oral route, and it is related to the size of the small intestine. The pulmonary route has a larger contact area, which makes absorption easier [62].
The major routes of MNPs-based drug administration are oral, inhalation, dermal and directly into the blood stream by intraperitoneal or intravenous injection [63].
Generally, blood half-life is shorter in rodents than in larger laboratory animals (e.g., rabbits or monkeys) and differs between intravenous and oral exposures. Oral, dermal, or inhalational absorption is low (≤ 5%), but may increase with smaller sizes, negative charge, and appropriate coatings [63].
Metallic NPs can be distributed throughout the body, primarily accumulating in the liver, spleen, and lymph node due to nonspecific uptake by reticuloendothelial cells, and could remain in the body for ≥ 6 months. Metallic NPs (≤ 100 nm) can cross the blood-brain barrier (BBB), favored by coating with BBB-permeable neuropeptides. Placental transfer depends on the stage of embryonic/placental maturation and surface composition of NP, and may be enhanced by coating with biocompatible molecules (e.g., ferritin or polyethylene glycol). Renal and biliary excretion is generally low due to persistent accumulation in tissues, but renal elimination could be substantially increased with smaller sizes and specific coatings (e.g., glutathione) [66].
DISTRIBUTION AND TOXICITY OF SILVER NANOPARTICLES
The absorbed AgNPs are dispersed throughout many systems, including the dermis, respiratory, spleen, digestive, urinary, nervous, immune, and reproductive systems. The primary distribution sites are the spleen, liver, kidneys, and lungs. Little AgNP deposition seen in the teeth and bones [63].
In addition to directly exposed tissues, NPs are also delivered to various organs with blood circulation. Nanosilver particles easily penetrate the body and cross biological barriers (BBB and blood-testis barrier) and can potentially produce a cytotoxic effect. Thus, non-specific distribution of AgNPs may produce cytotoxicities such as dermal toxicity, ocular toxicity, respiratory toxicity, hepatobiliary toxicity, neurotoxicity and reproductive toxicity, which limit the applications of AgNPs. The potential cytotoxicity of AgNPs depends on the routes of administration and the properties or characteristics of the AgNPs, such as the size, shape, and concentration (Fig. 6) [63].
However, the specific mechanisms of MNPs and AgNPs distribution and accumulation in various tissues and organs, as well as their potential toxicity, have not yet been sufficiently studied [5][62].
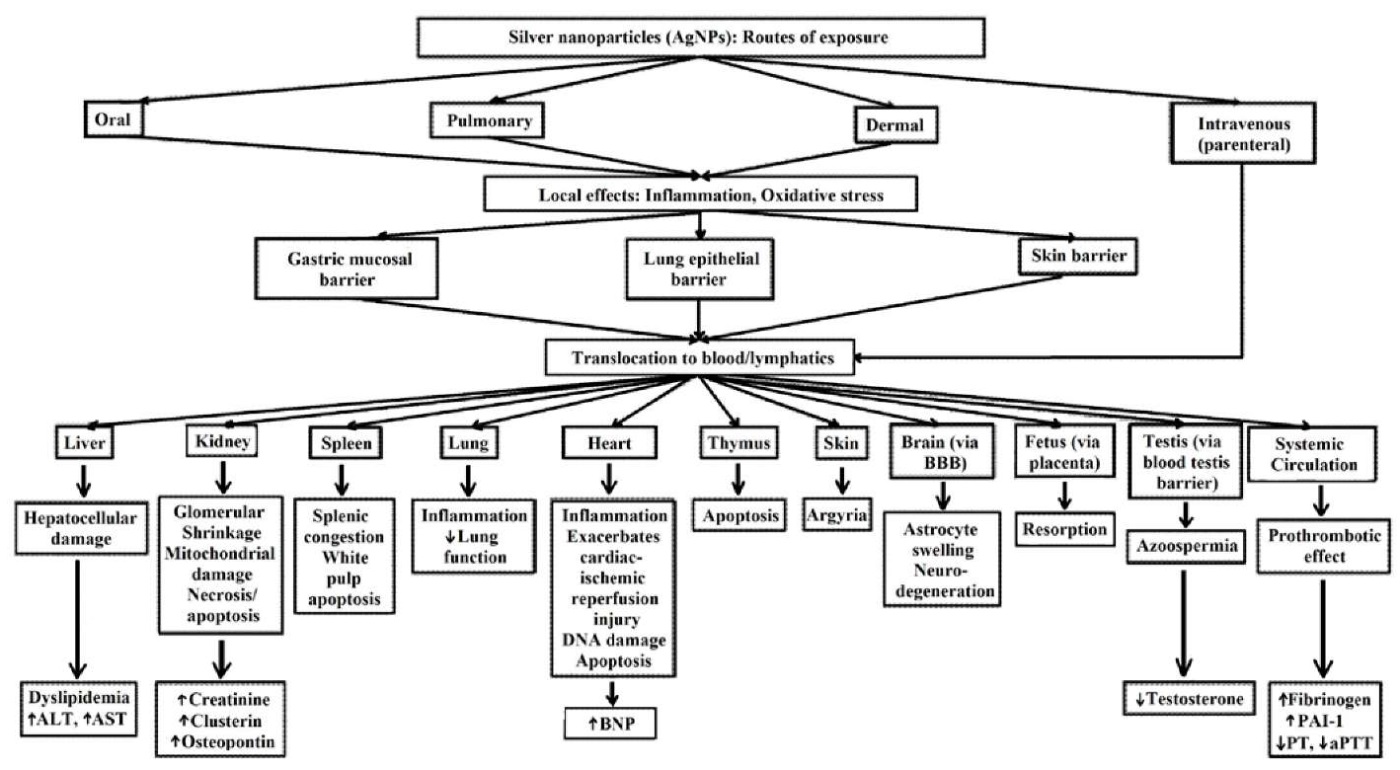
Fig. 6. Biodistribution and toxicity of silver nanoparticles for different exposure routes [63]
IMPACT OF SILVER NANOPARTICLES ON ANIMAL BODY
Silver nanoparticles may have different effects on the physiological parameters of animals, depending on the duration of use and doses of silver-based drugs. At the same time, both healthy and diseased animals always demonstrate changes in the biochemical and morphological blood parameters. Nanosilver mainly affects red blood cells and platelets, to a lesser extent affecting monocytes and leukocytes. Previously, O. A. Zeinalov et al. noted a moderate increase in platelet count and a decrease in white blood cell counts in healthy mice treated with high doses of highly dispersed metallic AgNPs [67]. Also E. M. Tsygankov et al. detected an increase in red blood cells and platelet counts in replacement flocks after using a cluster silver-based drug [68]. Study of 2021 found a significant decrease in white blood cell levels after using nano-dispersed silver to treat cows with serous mastitis, and a slight decrease in monocyte counts and an increase in hemoglobin levels were also observed [11]. When using highly dispersed nanosilver in mice with Newcastle disease, the monocyte count decreases, the mean corpuscular hemoglobin concentration decreases, and red blood cells, hemoglobin, and hematocrit levels increase [69].
Silver nanoparticles are particularly attractive for veterinary medicine as dietary supplements used to increase animal performance and immunity [10][11][12][13]. There are publications describing the promising use of virocidal agents based on nano- and organic silver to prevent Newcastle and Aujeszky’s diseases [69][70][71]. It is also well known that NPs in drinking water or dietary supplements exert anabolic effect; they can increase body weight and muscle mass [72][73][74][75].
However, as mentioned above, AgNPs mainly accumulate in the “filter organs” of the body, and can cross biological barriers. Toxic effects and cognitive impairment are noted after prolonged use of silver-containing drugs in animals, presumably due to the accumulation of AgNPs in the brain; and the use of silver-based drugs during mating, pregnancy and lactation of animals leads to a significant accumulation of AgNPs in tissues and organs not only of parents, but also of their offspring [76][77].
Thus, AgNPs administered in doses not exceeding 10 mg per 1 kg of body weight per day have biotic effects: they stimulate the respiratory function of the blood, increase red blood cells and hemoglobin levels; they stimulate the body defenses by increasing white blood cell count in the bloodstream [67][68][78]. Low doses and administration of nanosilver for maximum 30 days exert no significant effect on the gut microbiota and increase the animal performance [72][73][74][75]. The use of silver-containing drugs in high concentrations, as well as their prolonged use, negatively affects the mammalian body, and even can cause death [76][77][79].
Therefore, further in-depth studies of the biodistribution, compatibility and potential toxicity of NPs are still needed to facilitate the development of effective dietary supplements and safe drugs [5][60][62][63][66][79].
CONCLUSIONS
Based on the analysis of publications it can be concluded that.
- Over the past 20 years, a wide range of nanomaterials have been introduced in biomedicine, veterinary medicine and diagnostics. They are divided into organic and inorganic NPs. The latter include NPs of gold, silver, copper oxide, zinc oxide, magnesium oxide, iron oxide, titanium dioxide and aluminum.
- The use of NPs in veterinary practice has not yet found such widespread use as in public health, but it keeps expanding every year.
- AgNPs are of the greatest interest to Russian scientists, as they have long proven themselves as an antibacterial nanoagent.
- There are physical and chemical methods for NPs synthesis. They include reduction of silver ions (Ag+) and dispersal to nanoscale sizes.
- The antibacterial activity of AgNPs is influenced by their shape, size and surface charge.
- Currently, there are three types of silver-based drugs: colloidal (cationic), cluster and nulvalent (metallic).
- The biodistribution of MNPs is affected by the type of particles, their size, surface charge and coating, protein binding, as well as the exposure route and dose.
- The distribution of AgNPs does not differ from the MNPs pharmacokinetics, while nanoscale silver most often accumulates in the spleen, liver, kidneys and lungs, which potentially can be cytotoxic.
- Nanosilver administration in low doses and maximum for 30 days strengthens the immunity and improves the performance of animals, and prolonged use of AgNPs and/or administration in high concentrations contributes to the accumulation of silver in mammalian organs and tissues, exerting a toxic effect.
1. The World Health Organization. Antibiotic resistance. https://www.who.int/ru/news-room/fact-sheets/detail/antibiotic-resistance
2. https://scholar.google.com
3. https://wordstat.yandex.ru
4. On supervision of nanotechnology products and nanomaterial-based drugs: Regulation of the Chief Medical Officer of the Russian Federation 23.07.2007 No. 54. https://docs.cntd.ru/document/902056894
5. Procedure and organization of control over nanomaterials: Guidelines of 17.10.2011 MI 1.2.2966-11, htpps://docs.cntd.ru/document/1200095623
References
1. Akdoğan D., Güzel M., Genç Bahçe Y., Aksoy A., Akpınar O. Comparative antimicrobial susceptibility profiles of uropathogenic extended-spectrum ß-lactamase producing strains of Klebsiella pneumonia and Escherichia coli by the CLSI and EUCAST methodologies. Gazi Medical Journal. 2021; 32 (1): 88–93. http://dx.doi.org/10.12996/gmj.2021.16
2. ChernyavskayaYa. V., DenisovaT. P. Mekhanizmy deistviya nanochastits na organizmy = Mechanisms of nanoparticle action on the organism. Problemy teoreticheskoi i eksperimental’noi khimii: tezisy dokladov XXXI Rossiiskoi molodezhnoi nauchnoi konferentsiis mezhdunarodnym uchastiem, posvyashchennoi 90-letiyu so dnya rozhdeniya professora V. M. Zhukovskogo (Ekaterinburg, 20–23 aprelya 2021 g.) = Challenges of theoretical and experimental chemistry: abstracts of XXXI Russian scientific conference for early-career scientists devoted to 90th anniversary of the birth of professor V. M. Zhukovsky (Ekaterinburg, 20–23 April, 2021). Ekaterinburg: Ural University Press; 2021; 120. https://elar.urfu.ru/handle/10995/100016 (in Russ.)
3. Sadr S., Poorjafari Jafroodi P., Haratizadeh M. J., Ghasemi Z., Borji H., Hajjafari A. Current status of nano-vaccinology in veterinary medicine science. Veterinary Medicine and Science. 2023; 9 (5): 2294–2308. https://doi.org/10.1002/vms3.1221
4. Liew K. B., Janakiraman A. K., Sundarapandian R., Khalid S. H., Razzaq F. A., Ming L. C., et al. A review and revisit of nanoparticles for antimicrobial drug delivery. Journal of Medicine and Life. 2022; 15 (3): 328–335. https://pubmed.ncbi.nlm.nih.gov/35449993
5. Xu L., WangY. Y., Huang J., Chen C. Y., Wang Z. X., Xie H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics. 2020; 10 (20): 8996–9031. https://doi.org/10.7150/thno.45413
6. Woldeamanuel K. M., Kurra F. A., Roba Y. T. A review on nanotechnology and its application in modern veterinary science. International Journal of Nanomaterials, Nanotechnology and Nanomedicine. 2021; 7 (1): 026–031. http://doi.org/10.17352/2455-3492.000041
7. El-Sayed A., Kamel M. Advanced applications of nanotechnology in veterinary medicine. Environmental Science and Pollution Research. 2020; 27 (16): 19073–19086. https://doi.org/10.1007/s11356-018-3913-y
8. Alghuthaymi M. A., Hassan A. A., Kalia A., Sayed El Ahl R. M. H., El Hamaky A. A. M., Oleksak P., et al. Antifungal nano-therapy in veterinary medicine: current status and future prospects. Journal of Fungi. 2021; 7 (7):494. https://doi.org/10.3390/jof7070494
9. Kovalenko A. M., Tkachev A. V., Tkacheva O. L., Gutyj B. V., PrystupaO. I., Kukhtyn M. D., et al. Analgesic effectiveness of new nanosilver drug. UkrainianJournal of Ecology. 2020; 10 (1): 300–306. https://elibrary.ru/kfuixg
10. More P. R., Pandit S., Filippis A., Franci G., Mijakovic I., Galdiero M. Silver nanoparticles: bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms. 2023; 11 (2):369. https://doi.org/10.3390/microorganisms11020369
11. Nefedova E. V., Shkil N. N. Effect of silver nanoparticles on morphological, biochemical, and immunological parameters of cow blood with a serous form of mastitis. Russian Agricultural Sciences. 2021; 47 (Suppl. 1): S97–S100. https://doi.org/10.3103/S1068367422010128
12. Shkil N. N., Nefyodova E. V., Shkil N. A., Nozdrin G. A., Lazareva M. V., Rasputina O. V., Ryumkina I. N. Adjuvant properties of silver and dimethyl sulfoxide nanoparticles in studying antibacterial activity of antibiotics against E. coli. International Journal of Agriculture and Biological Sciences. 2020; 4 (5): 119–126. https://doi.org/10.5281/zenodo.4286955
13. Luceri A., Francese R., Lembo D., Ferraris M., Balagna C. Silver nanoparticles: Review of antiviral properties, mechanism of action and applications. Microorganisms. 2023; 11 (3):629. https://doi.org/10.3390/microorganisms11030629
14. Korolev D. V., Zakharova E. V., Evreinova N. V., Toropova Y. G., Pechnikova N. A., Sergienko E. S., Gareev K. G. The dynamics of the natural biodistribution of magnetic nanoparticles synthesized in various ways, when a single infusion to Wistar rats. Translational Medicine. 2016; 3 (4): 56–65. https://elibrary.ru/ymjgtt (in Russ.)
15. Shafiq M., Anjum S., Hano C., Anjum I., Abbasi B. H. An overview of the applications of nanomaterials and nanodevices in the food industry. Foods. 2020; 9 (2):148. https://doi.org/10.3390/foods9020148
16. Hassan S., Prakash G., Bal Öztürk A., Saghazadeh S., Sohail M. F., Seo J., et al. Evolution and clinical translation of drug delivery nanomaterials. Nano Today. 2017; 15: 91–106. https://doi.org/10.1016/j.nantod.2017.06.008
17. Turner C. T., McInnes S. J. P., Voelcker N. H., Cowin A. J. Therapeutic potential of inorganic nanoparticles for the delivery of monoclonal antibodies. Journal of Nanomaterials. 2015; 2015 (1):309602. https://doi.org/10.1155/2015/309602
18. Dizaj S. M., Jafari S., Khosroushahi A. Y. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Research Letters. 2014; 9 (1):252. https://doi.org/10.1186/1556-276x-9-252
19. Poon C., Gallo J., Joo J., Chang T., Bañobre-López M., Chung E. J. Hybrid, metal oxide-peptide amphiphile micelles for molecular magnetic resonance imaging of atherosclerosis. Journal of Nanobiotechnology. 2018; 16 (1):92. https://doi.org/10.1186/s12951-018-0420-8
20. Stanovaya A., Terekhova M., Abashkin V., Odabashi M., Shcherbin D. Nanoparticles in biology and medicine. Science and Innovations. 2022; (11): 78–83. https://doi.org/10.29235/1818-9857-2022-11-78-83 (in Russ.)
21. Mughal B., Zaidi S. Z. J., Zhang X., Hassan S. U. Biogenic nanoparticles: synthesis, characterisation and applications. Applied Sciences. 2021; 11 (6):2598. https://doi.org/10.3390/app11062598
22. Igonin A. S. Primenenie nanochastits metallov v meditsine = Use of metal nanoparticles in medicine. Khimiya i khimicheskoe obrazovanie XXI veka: sbornik materialov VI Vserossiiskoi studencheskoi konferentsii s mezhdunarodnym uchastiem, posvyashchennoi 310-letiyu so dnya rozhdeniya M. V. Lomonosova (Sankt-Peterburg, 22–26 marta 2021 g.) = Chemistry and education in chemistry in XXI century: proceedings of VI All-Russian Student Conference with international participation devoted to 310th anniversary of the birth of M. V. Lomonosov (Saint Petersburg, 22–26 March, 2021). Saint Petersburg: HerzenUniversity; 2021; 70–71. https://elibrary.ru/ckyhst (in Russ.)
23. Kapranova K. A. Primenenie nanochastits metallov v meditsine = Application of metal nanoparticles in medicine. Innovatsionnye tekhnologii, ekonomika i menedzhment v promyshlennosti: sbornik nauchnykh statei III mezhdunarodnoi nauchnoi konferentsii (Volgograd, 31 oktyabrya 2022 g.) = Innovation technologies, economics and management in industry: proceedings of III International Scientific Conference (Volgograd, 31 October, 2022). Volgograd: LLC “Aktualnost.RF”; 2022; 4–7. https://elibrary.ru/gkrurh (in Russ.)
24. Agafonov D. A. Nanochastitsy serebra i zolota kak lekarstvennye sredstva = Silver and gold nanoparticles astherapeutic drugs. Aktual’nye voprosy farmatsevticheskikh i estestvennykh nauk: sbornik statei Vserossiiskoi studencheskoi nauchno-prakticheskoi konferentsii s mezhdunarodnym uchastiem (Irkutsk, 21–26 sentyabrya 2020 g.) = Topical issues of pharmaceutical and natural sciences: proceedings of All-Russian student scientific and practical conference with international participation (Irkutsk, 21–26 September, 2020). Irkutsk: Irkutsk State Medical University; 2020; 162–164. https://elibrary.ru/jcdbzr (in Russ.)
25. Dadfar S. M., Roemhild K., Drude N. I., von Stillfried S., Knüchel R., Kiessling F., Lammers T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Advanced Drug Delivery Reviews. 2019; 138: 302–325. https://doi.org/10.1016/j.addr.2019.01.005
26. Ameen F., AlYahya S., Govarthanan M., ALjahdali N., Al-Enazi N., Alsamhary K., et al. Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. Journal of Molecular Structure. 2020; 1202:127233. https://doi.org/10.1016/j.molstruc.2019.127233
27. Hooda H., Singh P., Bajpai S. Effect of quercitin impregnated silver nanoparticle on growth of some clinical pathogens. Materials Today: Proceedings. 2020; 31 (Pt. 4): 625–630. https://doi.org/10.1016/j.matpr.2020.03.530
28. Kalimuthu K., Cha B. S., Kim S., Park K. S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: A review. Microchemical Journal. 2020; 152:104296. https://doi.org/10.1016/j.microc.2019.104296
29. Siddiqi K. S., Husen A. Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. Journal of Trace Elements in Medicine and Biology. 2017; 40: 10–23. https://doi.org/10.1016/j.jtemb.2016.11.012
30. Liu S., Yu B., Wang S., Shen Y., Cong H. Preparation, surface functionalization and application of Fe3 O4 magnetic nanoparticles. Advancesin Colloid and Interface Science. 2020; 281:102165. https://doi.org/10.1016/j.cis.2020.102165
31. Suman P. H. Electrical properties of tin oxide materials. In: Tin Oxide Materials: Synthesis, Properties, andApplications. Ed. byM. O. Orlandi. Elsevier; 2020; 41–60. https://doi.org/10.1016/B978-0-12-815924-8.00003-7
32. Nunes D., Pimentel A., Gonçalves A., Pereira S., Branquinho R., Barquinha P., et al. Metal oxide nanostructures for sensor applications. Semiconductor Science and Technology. 2019; 34 (4):043001. https://doi.org/10.1088/1361-6641/ab011e
33. Safina L. A., ZifirovaYu. S. Analiz primeneniya nanomaterialov v meditsine = Analysis of nanomaterial application in medicine. Novye tekhnologii i materialy legkoi promyshlennosti: sbornik statei XVІ Vserossiiskoi nauchno-prakticheskoi konferentsiyi s elementami nauchnoi shkoly dlya studentov i molodykh uchenykh (Kazan’, 19–23 maya 2020 g.) = New technologies and materials of light industry: Proceedings of XVІ All-Russian Scientific and Practical Conferencewith elements ofscientific schoolforstudents and early career scientists (Kazan, 19–23 May, 2020). Kazan: Kazan National Research Technological University; 2020; 120–124. https://elibrary.ru/rnanhb (in Russ.)
34. Dukhova Yu. S., Evdokimova A. V. Organo-neorganicheskie nanomaterialy na osnove oksidov metallov i nanotsellyulozy: poluchenie isvoistva = Organic/inorganic nanomaterials based on metal oxides and nanocellulose: production and properties. Molodye uchenye – razvitiyu Natsional’noi tekhnologicheskoi initsiativy (POISK). 2021; (1): 301–302. https://elibrary.ru/peopvx (in Russ.)
35. Javadi S. M. Applications of ZnOand MgOnanoparticlesin reducing Zinc pollution level in rubber manufacturing processes: A review. Current Biochemical Engineering. 2020; 6 (2): 103–107. https://doi.org/10.2174/2212711906666200224105931
36. Poreddy R., Engelbrekt C., Riisager A. Copper oxide as efficient catalyst for oxidative dehydrogenation of alcohols with air. Catalysis Science & Technology. 2015; 5 (4): 2467–2477. https://doi.org/10.1039/c4cy01622j
37. Matsakova E. G., Simakova D. I. Nanoparticles manifesting antibacterial effects: properties, production, mechanism of action, and applications. Nanotechnologiesin Russia. 2020; 15 (2): 236–240. https://doi.org/10.1134/S1995078020020159
38. Martynova E. U., Kozlov E. N., Mukha D. V. Nanoparticles: potentialities of their use in medicine and veterinary. Uspehi sovremennoj biologii. 2012; 132 (5): 435–447. https://elibrary.ru/pgxdwz (in Russ.)
39. Tang S., Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Advanced Healthcare Materials. 2018; 7 (13):e1701503. https://doi.org/10.1002/adhm.201701503
40. Doitsh G., Galloway N. L., Geng X., Yang Z., Monroe K. M., ZepedaO., et al. Cell death by pyroptosis drives CD4 T-cell depletion inHIV-1 infection. Nature. 2014; 505 (7484): 509–514. https://doi.org/10.1038/nature12940
41. Rai M., Deshmukh S. D., Ingle A. P., Gupta I. R., Galdiero M., Galdiero S. Metal nanoparticles: The protective nanoshield against virusinfection. Critical Reviews in Microbiology. 2016; 42 (1): 46–56. https://doi.org/10.3109/1040841x.2013.879849
42. Lin Q., Lim J. Y. C., Xue K., Yew P. Y. M., Owh C., Chee P. L., Loh X. J. Sanitizing agents for virus inactivation and disinfection. View. 2020; 1 (2):e16. https://doi.org/10.1002/viw2.16
43. Khodashenas B., Ghorbani H. R. Synthesis of silver nanoparticles with differentshapes. Arabian Journal of Chemistry. 2019; 12 (8): 1823–1838. https://doi.org/10.1016/j.arabjc.2014.12.014
44. Rai M., Kon K., Gade A., Ingle A., Nagaonkar D., Paralikar P., da Silva S. S. Antibiotic resistance: Can nanoparticlestackle the problem? In: Antibiotic Resistance: Mechanisms andNewAntimicrobial Approaches. Eds. K. Kon, M. Rai. Academic Press; 2016; Chapter 6: 121–143. https://doi.org/10.1016/B978-0-12-803642-6.00006-X
45. Jan T., Azmat S., Mansoor Q., Waqas H. M., Adil M., Ilyas S. Z., et al. Superior antibacterial activity of ZnO-CuO nanocomposite synthesized by a chemical Co-precipitation approach. Microbial Pathogenesis. 2019; 134:103579. https://doi.org/10.1016/j.micpath.2019.103579
46. Helmlinger J., Sengstock C., Groß-Heitfeld C., Mayer C., SchildhauerT. A., Köller M., Epple M. Silver nanoparticles with differentsize and shape: equal cytotoxicity, but different antibacterial effects. RSC Advances. 2016; 6 (22): 18490–18501. https://doi.org/10.1039/c5ra27836h
47. Dong Y., Zhu H., ShenY., Zhang W., Zhang L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio natriegens. PLoS ONE. 2019; 14 (9):e0222322. https://doi.org/10.1371/journal.pone.0222322
48. Pal S., Tak Y. K., Song J. M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and Environmental Microbiology. 2007; 73 (6): 1712–1720. https://doi.org/10.1128/aem.02218-06
49. Jones N., Ray B., Ranjit K. T., Manna A. C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiology Letters. 2008; 279 (1): 71–76. https://doi.org/10.1111/j.1574-6968.2007.01012.x
50. Sirelkhatim A., Mahmud S., Seeni A., Kaus N. H. M., Ann L. C., Bakhori S. K. M., et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Letters. 2015; 7 (3): 219–242. https://doi.org/10.1007/s40820-015-0040-x
51. Liu J., Wang Y., Ma J., Peng Y., Wang A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. Journal of Alloys and Compounds. 2019; 783: 898–918. https://doi.org/10.1016/j.jallcom.2018.12.330
52. Roelofs D., Makama S., de Boer T. E., Vooijs R., van Gestel C. A. M., van den Brink N. W. Surface coating and particle size are main factors explaining the transcriptome-wide responses of the earthworm Lumbricus rubellus to silver nanoparticles. Environmental Science: Nano. 2020; 7 (4): 1179–1193. https://doi.org/10.1039/c9en01144g
53. Hu C., Wang L.-L., Lin Y.-Q., Liang H.-M., Zhou S.-Y., Zheng F., et al. Nanoparticlesfor the treatment of oral biofilms: currentstate, mechanisms, influencing factors, and prospects. Advanced Healthcare Materials. 2019; 8 (24):e1901301. https://doi.org/10.1002/adhm.201901301
54. Кoshelev K. K. Silver-containing preparation: analysis of advantages and disadvantages. Raw Materials & Packaging. 2012; (8). https://cosmetic-industry.com/serebrosoderzhashhie-preparaty-analiz-preimushhestv-i-nedostatkov.html (in Russ.)
55. Shumakova A. A., Smirnova V. V., Tananova O. N., Trushina E. N., Kravchenko L. V., Aksenov I. V., et al. Toxicological sanitary characterization of silver nanoparticles introduced in gastrointestinal tract of rats. Problems of Nutrition. 2011; 80 (6): 9–18. https://www.voprosy-pitaniya.ru/ru/jarticles_diet/68.html?SSr=300134b42314ffffffff27c__07e70c030e1e35-3b9d (in Russ.)
56. Adegbeye M. J., Elghandour M. M. M. Y., Reddy P. R. K., Alqaisi O., Oloketuyi S., Salem A. Z. M., Asaniyan E. K. Potential of silver nanoparticles for veterinary applications in livestock performance and health. In: Silver Nanomaterials for Agri-Food Applications. Ed. by K. A. Abb-Elsalam. Elsevier; 2021; Chapter 27: 657–683. https://doi.org/10.1016/B978-0-12-823528-7.00022-6
57. Murugan K., Wang L., Anitha J., Dinesh D., Amuthavalli P., Vasanthakumaran M., et al. Insecticidal effect of chitosan reduced silver nanocrystals against filarial vector, Culex quinquefasciatus and cotton bollworm, Helicoverpa armigera. In: Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture. Woodhead Publishing; 2021; Chapter 19: 469–486. https://doi.org/10.1016/B978-0-12-820092-6.00019-7
58. Prasad R. D., Prasad S. R., Shrivastav O. P., Kanthe A. R., Waghmare S. R., Shaikh V. S., et al. Biogenic synthesis of nano-silver and their anti-microbial efficacy. ES Food & Agroforestry. 2023; 11:836. https://doi.org/10.30919/esfaf836
59. Shevchenko L., Mitsevsky M., Slobodyanyuk N., Kondratiuk V., Ustymenko I., Ivaniuta A., et al. Metabolic status of calves using nanosilver with milk diet. Journal of Hygienic Engineering & Design. 2023; 43:245. https://keypublishing.org/jhed/wp-content/uploads/2023/08/17.-Abstract-Larysa-Shevchenko.pdf
60. Gelperina S. E., Shvets V. I. Drug delivery systems based on polymeric nanoparticles. Biotechnology in Russia. 2009; (3): 1–21. https://elibrary.ru/miegxy
61. Zhu D., Long Q., Xu Y., Xing J. Evaluating nanoparticles in preclinical research using microfluidic systems. Micromachines. 2019; 10 (6):414. https://doi.org/10.3390/mi10060414
62. JosephT. M., Kar Mahapatra D., Esmaeili A., Piszczyk Ł., Hasanin M. S., Kattali M., et al. Nanoparticles: Taking a unique position in medicine. Nanomaterials. 2023; 13 (3):574. https://doi.org/10.3390/nano13030574
63. Ferdous Z., Nemmar A. Health impact of silver nanoparticles: a review ofthe biodistribution and toxicity following variousroutes of exposure. International Journal of Molecular Sciences. 2020; 21 (7):2375. https://doi.org/10.3390/ijms21072375
64. Cho E. C., Zhang Q., Xia Y. The effect ofsedimentation and diffusion on cellular uptake of gold nanoparticles. Nature Nanotechnology. 2011; 6 (6): 385–391. https://doi.org/10.1038/nnano.2011.58
65. Wang H., Chen B., He M., Li X., Chen P., Hu B. Study on uptake of gold nanoparticles by single cells using droplet microfluidic chip-inductively coupled plasma mass spectrometry. Talanta. 2019; 200: 398–407. https://doi.org/10.1016/j.talanta.2019.03.075
66. Kozics K., Sramkova M., Kopecka K., Begerova P., Manova A., Krivosikova Z., et al. Pharmacokinetics, biodistribution, and biosafety of PEGylated gold nanoparticlesin vivo. Nanomaterials. 2021; 11 (7):1702. https://doi.org/10.3390/nano11071702
67. Zeinalov O. А., Kombarova S. P., Bagrov D. V., Petrosyan M. A., Tolibova G. H., Feofanov A. V., Shaitan K. V. About the influence of silver nanoparticles on living organisms physiology. Reviews on Clinical Pharmacology and Drug Therapy. 2016; 14 (4): 42–51. https://doi.org/10.17816/RCF14442-51 (in Russ.)
68. Tsygankov E. M., Men’kova A. A., Andreev A. I. Hematologic parameters of the blood of the reconstructer young bird of the bird underthe influence of drug Argodez. Scientific Notes Kazan Bauman State Academy of Veterinary Medicine. 2017; 232 (4): 150–154. https://elibrary.ru/zvrhxl (in Russ.)
69. Sumarokova A. D., Statsevich L. N., AfonyushkinV. N., KoptevV. Y., CherepushkinaV. S. Changesin hematological parameters when using nanosilver preparations in laboratory ICR mice infected with the Newcastle disease virus. International Journal of Veterinary Medicine. 2024; (2): 31–41. https://doi.org/10.52419/issn2072-2419.2024.2.31 (in Russ.)
70. Sumarokova A. D., Afonyushkin V. N., Mironova T. E., Cherepushkina V. S., Afonyushkin A. V., Stastevich L. N., SilnikovV. N. Study ofthe biological activity of silver preparations on an organismal model of Newcastle disease infection. Siberian Herald of Agricultural Science. 2024; 54 (7): 96–105. https://doi.org/10.26898/0370-8799-2024-7-10 (in Russ.)
71. Krasochko P. A., Borisovets D. S., Stankut A. E., Krasochko I. A., Zuikevich T. A. Evaluation of antiviral properties of silver nanoparticles in in vitro and in vivo systems. Veterinarnyi zhurnal Belarusi. 2024; (1): 36–40. https://repo.vsavm.by/handle/123456789/24356 (in Russ.)
72. Shamsutdinova I. R., Derkho M. A. Peculiarities of biological impact of silver nanoparticles on the animals’ body. Izvestia Orenburg State Agrarian University. 2016; (1): 202–205. https://elibrary.ru/vpfdrd (in Russ.)
73. Saleh A. A., El-Magd M. A. Beneficial effects of dietary silver nanoparticles and silver nitrate on broiler nutrition. Environmental Science and Pollution Research. 2018; 25 (27): 27031–27038. https://doi.org/10.1007/s11356-018-2730-7
74. Zaoui Y., Belanche A., Ben-Jeddou K., Jiménez M. S., Fondevila G., Fondevila M. Effect of the dietary administration pattern of silver nanoparticles on growth performance, biodiversity of digestive microbiota and tissue retention in broiler chickens. Animal Feed Science and Technology. 2024; 309:115888. https://doi.org/10.1016/j.anifeedsci.2024.115888
75. Elkloub K., Moustafa M. El., Ghazalah A. A., Rehan A. A. A. Effect of dietary nanosilver on broiler performance. International Journal of Poultry Science. 2015; 14 (3): 177–182. https://doi.org/10.3923/ijps.2015.177.182
76. Antsiferova A. A., Kashkarov P. K., Koval’chuk M. V. Effect of different forms of silver on biological objects. Nanotechnology Reports. 2022; 17 (2): 155–164. https://doi.org/10.1134/S2635167622020021
77. Zinicovscaia I., Ivlieva A. L., Petritskaya E. N., Rogatkin D. A. Unexpected reproductive effect of prolonged oral administration of silver nanoparticles in laboratory mice. Human Ecology. 2020; 27 (10): 23–30. https://doi.org/10.33396/1728-0869-2020-10-23-30 (in Russ.)
78. Shamsutdinova I. R., Derkho M. A. Changes of blood morphological parameters of laboratory animals when introducing silver nanoparticles per os. Agro-Industrial Complex of Russia. 2015; 73: 166–170. https://elibrary.ru/ulfokj (in Russ.)
79. Bakowski M., Kiczorowska B., Samolińska W., Klebaniuk R., Lipiec A. Silver and zinc nanoparticles in animal nutrition – A review. Annals of Animal Science. 2018; 18 (4): 879–898. https://doi.org/10.2478/aoas-2018-0029
About the Authors
A. D. SumarokovaRussian Federation
Anastasia D. Sumarokova, Postgraduate Student
160 Dobrolyubova str., Novosibirsk 630039
L. N. Statsevich
Russian Federation
Lyudmila N. Statsevich, Cand. Sci. (Biology), Associate Professor
160 Dobrolyubova str., Novosibirsk 630039
Review
For citations:
Sumarokova A.D., Statsevich L.N. Metal nanoparticles, silver nanoparticles and their impact on human and animal health (review). Veterinary Science Today. 2025;14(1):90-100. https://doi.org/10.29326/2304-196X-2025-14-1-90-100



































