Scroll to:
Optimization of freeze-drying process for anti-Chlamydia hyperimmune serum
https://doi.org/10.29326/2304-196X-2025-14-1-82-89
Abstract
Introduction. The distribution are a of Chlamydia infection in livestock and wild animals currently extends across almost all continents. At present, initial diagnosis, screening tests and certain stages of epizootiological investigations aimed at Chlamydia carrier detection are conducted in the Russian Federation using the “Antigen and Serum Kit for Serological Diagnosis of Chlamydiosis in Livestock”. It is important in theproduction of diagnostics to ensure stability of different test kit components during their storage and transportation. One way of addressing this issue is to stabilize diagnosticum components by freeze-drying.
Objective. The study was aimed at optimization of freeze-drying process for specific anti-Chlamydia serum, the serum assessment for compliance with characteristics laid down in the technical specifications for the test kit control and testing of the serum for its stability.
Materials and methods. The serum was prepared using blood from sheep immunized with emulsion vaccine based on Chlamydia psittaci AMK-16 strain. Prior tofreeze-drying, the hyperimmune sera were subjected to freezing to a temperature of minus 60 °С. The sera were freeze-dried using a Scientz 30F freeze-dryer (China). Two freeze-drying procedures with different temperature conditions and chamber pressures were applied. The resulting sera were tested for compliance with the technical specifications for thediagnostic test kit. The freeze-driedsera wereput intostorage for 24 months andtested with complement fixation test during this period.
Results. Based on the test results, the freeze-drying procedure employing a lower pressure and the highest heating temperature was found to be the most effective for the specific sera. The serum tests for compliance with characteristics laid down in the technical specifications for the test kit showed that the serum quality met all relevant requirements. The stability test results demonstrated that the hyperimmune serum freeze-dried using the improved procedure remains specific during 24 months.
Conclusion. The work performed allowed for optimization of freeze-drying process for specific anti-Chlamydia serum intended for the diagnostic test kit. The resulting serum is fully compliant with characteristics laid down in thetechnical specifications for the diagnosticum. It was established that the freeze-dried serum shelf life is at least two years, during this period the serum retains its activity and physico-chemical properties.
Keywords
For citations:
Evstifeev V.V., Yakovlev S.I., Khusainov F.M., Akbashev I.R., Sadykova S.V. Optimization of freeze-drying process for anti-Chlamydia hyperimmune serum. Veterinary Science Today. 2025;14(1):82-89. https://doi.org/10.29326/2304-196X-2025-14-1-82-89
INTRODUCTION
Chlamydiosis is an infectious disease common for animals and humans. It is caused by gram–negative bacteria of the family Chlamydiaceae, genus Chlamydia [1][2][3][4]. Once in the body of an animal (cattle, sheep, pigs, horses, rodents, birds, cats, dogs and many others), Chlamydia affects various body systems [5] including the immune system, which, in turn, facilitates subsequent co-infection with other pathogens [1]. The concurrent clinical signs of chlamydiosis and other infections in the animals hamper the accurate and timely diagnosis [6]. Chlamydia infection more frequently takes a chronic course, which also hinders the timely detection of infected animals after the introduction of the pathogen to the herd [7].
The distribution area of Chlamydia infection in livestock and wild animals currently extends across almost all continents and therefore its diagnosis is an urgent issue [8][9].
At present, initial diagnosis, screening tests and certain stages of epizootiological investigations aimed at Chlamydia carrier detection are conducted in the Russian Federation using the “Antigen and Serum Kit for Serological Diagnosis of Chlamydiosis in Livestock” (ROSS RU D-RU.RA01.V.19342/23) [10][11].
It is important in the production of diagnostica to ensure stability of biological properties of various test kit components during their manufacture, storage and transportation [12][13]. One way of addressing this issue is to stabilize the protein molecules of different diagnosticum components by freeze-drying [14][15][16].
The “Antigen and Serum Kit for Serological Diagnosis of Chlamydiosis in Livestock” manufactured by the Federal Center for Toxicological, Radiation and Biological Safety (Russia) contains two antigens (specific and control) and two animal sera (positive and negative to Chlamydia antigen). All components of the kit are freeze-dried [10].
During the test kit development, specific freeze-drying conditions were selected for each component of the kit. It should be noted that the freeze-drying procedure should be developed for each particular freeze-dryer depending on its design and characteristics, and freeze-drying parameters may vary for each component.
Scientz 30F freeze-dryer (China) was purchased as part of renovation of the technical facilities of the diagnostic test kit production site. It has more powerful vacuum pump and refrigeration unit. This has allowed for drying time reduction with the batch size remaining the same. Moreover, owing to the electronic control unit for drying parameters available in the freeze-dryer, the temperature and vacuum control ranges have become more accurate that makes it possible to set more process control points. Therefore, the drying procedure used previously for the old less powerful machine lacking precise control of the process parameters became unacceptable. New parameters and criteria for freeze-drying process control had to be developed and optimized. The scientific novelty of the work lies in the development of new parameters for freeze-drying of specific anti-Chlamydia serum using new equipment while maintaining the serum characteristics laid down in the technical specifications.
The study was aimed at optimization of freeze-drying process for specific anti-Chlamydia serum, the serum assessment for compliance with characteristics laid down in the technical specifications for the test kit control and the serum testing for its stability.
MATERIALS AND METHODS
The work was carried out at the Laboratory for Animal Viral Diseases, Federal Center for Toxicological, Radiation and Biological Safety.
Strain. Chlamydia psittaci AMK-16 strain with infectious titre of 10–5.4 LD50/0.3 mL deposited to the Microorganism Strain Collection of the Federal Center for Toxicological, Radiation and Biological Safety (reference No. 11 of 5 September 2017) was used for the inactivated antigen preparation.
Biological models. To prepare the antigen, Chlamydia was cultivated in 6–7-day-old embryonated chicken eggs. Romanov sheep aged 1.5 years with a live weight of 45–50 kg were used to prepare hyperimmune sera.
During the experiment, the animals were handled in compliance with Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
Test kits. The complement fixation test (CFT) was carried out using the “Antigen and Serum Kit for the serological diagnosis of Chlamydia in farm animals” (Federal Center for Toxicological, Radiation and Biological Safety, Kazan).
Equipment. The hyperimmune sera were freeze-dried using a Scientz 30F freeze dryer (China).
Methods. The antigen for immunization of the sheep was prepared from infected yolk membranes of embryonated chicken eggs that died on days 4–7 after infection by homogenizing them in phosphate buffered saline (pH 7.2) at a ratio of 1:9 with subsequent differential centrifugation to remove ballast substances, inactivation with formalin and concentration [17].
The serum was prepared from the blood collected from the sheep immunized with specific Chlamydia antigen emulsified in the original oil-lanolin adjuvant. The sera were tested for their antigenic activity with CFT using specific Chlamydia antigen in accordance with TU 9388-020-00492374-2007.
The sheep were bled under anesthesia. The blood was taken from the jugular vein. The blood was collected in sterile glass vessels, the inner surface of which was moistened with saline solution. To separate the serum, the vessels with blood were placed in a thermostat for 40–60 minutes, then blood clots were separated from the walls of the vessels, and the vessels were placed in a refrigerator at a temperature of 4 °C for 24 hours. The separated serum was decanted to remove the clots, centrifuged at 3.5 thousand rpm for 20 minutes to remove red blood cells and then preserved with boric acid.
The resulting sera were filled into paired glass ampoules (OST 64-2-485-85, Russia), 1.0 cm³ per ampoule, using a BioHit single-channel pipette (Finland). In total, three batches of hyperimmune ovine sera were prepared for the study.
All sera were divided into two equal parts and freeze-dried using two different freeze-drying procedures.
Before freeze-drying, the sera in ampoules were frozen in a freeze dryer chamber at a temperature of minus 60 °C for 14 hours.
The freeze-dried sera were put for storage for 24 months. at a temperature of 18–22 °C. The freeze-dried hyperimmune sera were tested with CFT every month to assess its activity and to determine the shelf life.
The sera were tested for their antigenic activity with CFT before and after freeze-drying in accordance with the “Instruction for the use of the “Antigen and Serum Kit for Serological Diagnosis of Chlamydiosis in Livestock” approved by the Director of the Federal Center for Toxicological, Radiation and Biological Safety on 19 May 2016 (ROSS RU D-RU.RA01.V.19342/23).
The test was carried out in a volume of 1.0 cm³ in Florinsky test tubes. The working dose of the antigen was used for the test. The sera were inactivated during 30 minutes and titrated by preparing 2-fold dilutions (starting from 1:5). Before test, the complement was titrated in the hemolytic system using its doubled dose; immune anti-Chlamydia and known negative serum as well as control antigen were used to control the test specificity. The hemolytic system was prepared using a 2.5% mixture of washed ram red blood cells and standard hemolytic serum at doubled titer. The test was carried out on water bath at a temperature of 37 °C. A 1:10 serum dilution was taken as the diagnostic titre, and a 1:5 dilution was considered inconclusive [18].
The resulting serum was to comply with the characteristics laid down in TU 9388-020-00492374-2007.
The prepared serum was tested for the following parameters: appearance, colour, presence of extraneous matter and mold, moisture content, solubility, CFT activity and specificity as well as shelf life.
The serum was visually examined for its appearance, colour and presence of extraneous matter and mold.
To test the freeze-dried serum for its solubility, saline solution was added to the ampoules with the serum, 1.0 cm³ per ampoule, the ampoules were shaken and the time until the freeze-dried serum completely dissolved was recorded.
The moisture content in the freeze-dried serum was determined in accordance with the rules laid down in GOST 24061-2012. Freeze-dried sera weighing 0.1 g were ground into powder. The prepared samples were evenly distributed over the bottom of a preliminarily weighed weighing vessel. After weighing, the weighing vessels containing the serum samples were placed in a drying cabinet and kept at a temperature of 105 °C for 60 minutes. Then, they were cooled and weighed.
Moisture content was calculated according the following formula [19]:
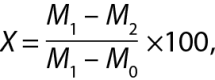
where X is moisture content, %;
M1 is the weight of the weighing vessel containing the serum sample before drying, g;
M2 is the weight of the weighing vessel containing the serum sample after drying, g;
M0 is the weight of the weighing vessel without the serum sample, g.
STUDY RESULTS
During the first stage of the study, the prepared sheep sera were tested for their antigenic activity. The sera of all the three batches reacted with Chlamydia antigen at a titre of 1:160 when tested with CFT. Then the sera filled in ampoules, 1.0 cm³ per ampoule, and frozen to a temperature of minus 60 °C, were freeze-dried using two different procedures. Temperature and vacuum pressure values during hyperimmune sheep serum freeze-drying according to procedure 1 are shown in Figure 1.
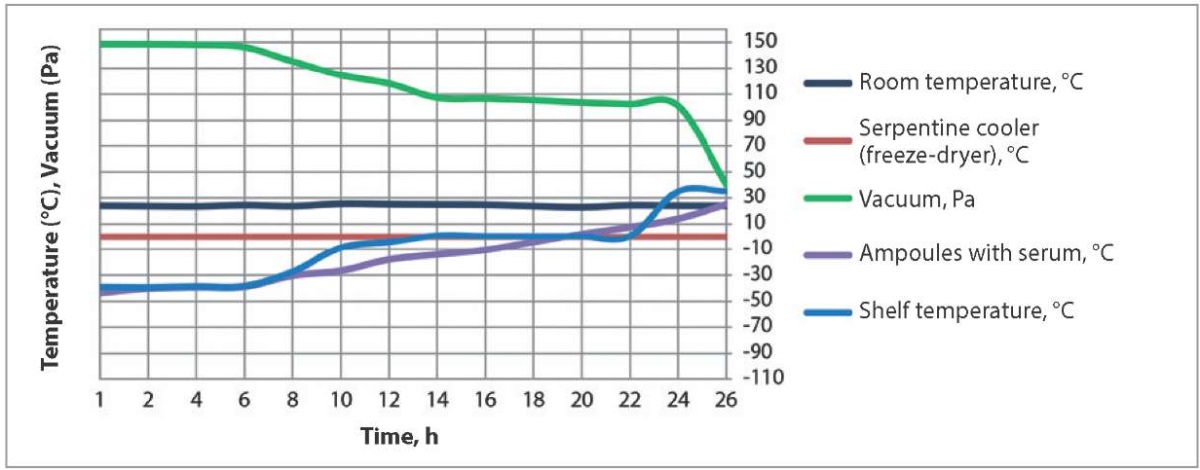
Fig. 1. Temperature conditions and vacuum pressure during hyperimmune serum freeze-drying using procedure 1
This freeze-drying procedure included keeping sera under vacuum for 26 hours with a gradual pressure decrease from 148 to 100 Pa within the first 25 hours and to 40 Pa within the subsequent one hour. The shelves were cooled during the first 7 hours. Then, during the next 6 hours (hour 8 to 13 after the freeze-drying start), the shelves were heated to a temperature of 0.2–0.8 °C, and the temperature of the shelves was kept within these limits for hour 14 to 21 after the freeze-drying start. The shelves of the freeze-dryer were switched to heating mode (up to a temperature of 35 °C) on hour 22 of the freeze-drying. The serum was dried under such conditions for another 4 hours until its temperature in the ampoules reached 25 °C.
Temperature and vacuum pressure values during hyperimmune sheep serum freeze-drying using procedure 2 are shown in Figure 2.
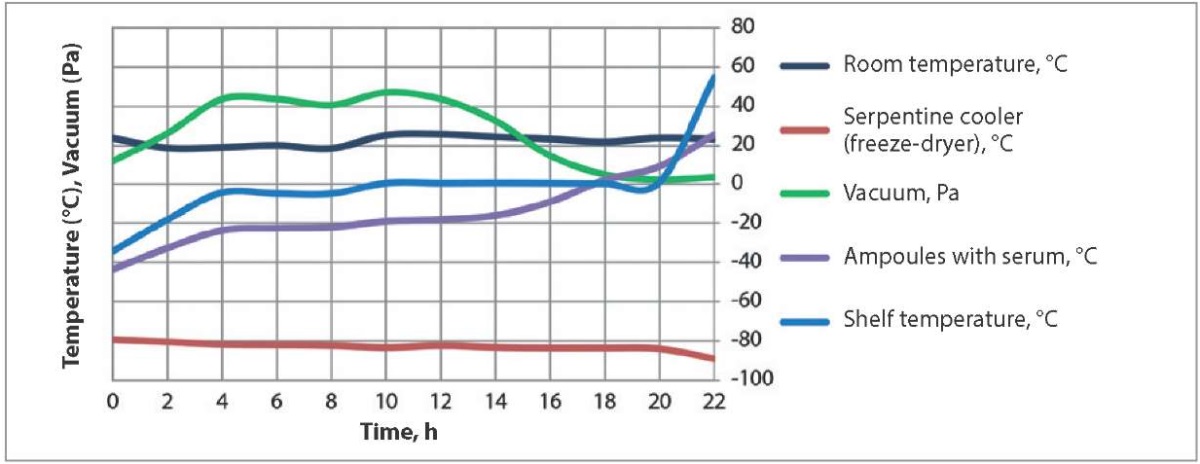
Fig. 2. Temperature conditions and vacuum pressure during hyperimmune serum freeze-drying using procedure 2
The difference between the procedures was that under procedure 2 the vacuum pressure was maintained at 11 Pa during the 1st hour after placing the sera into the freeze dryer. During the next 3 hours, the pressure in the freeze dryer chamber gradually increased to 43 Pa and was maintained at this level for the next 5 hours. From hour 9 to hour 11 after the freeze-drying start the pressure in the chamber was increased to 47 Pa. From hour 13 to hour 20 of the freeze-drying process, the pressure in the chamber was decreased to 2.2 Pa. The freeze dryer shelves were cooled for 1 hour. From hour 2 to hour 9 the shelves were gradually heated to a temperature of 0.6 °C. Temperature of the shelves was kept at this level for the next 10 hours (starting from hour 10 to hour 20 after the freeze-drying start). During the last 2 hours of the serum freeze-drying, the freeze dryer shelves were heated from 1 to 54 °C until the serum temperature in the ampoules reached 25 °C.
Figure 3 presents the photographs of hyperimmune sera freeze-dried using the two procedures described above.

Fig. 3. Appearance of sera from the same batch freeze-dried using two different procedures
The table shows the results of the freeze-dried serum tests for compliance with characteristics laid down in technical specifications.
Table
Physico-chemical and biological parameters of freeze-dried anti-Chlamydia hyperimmune sera and their compliance with characteristics laid down in technical specifications
|
Parameter |
Serum characteristics in accordance with TU 9388-020-00492374-2007 |
Serum freeze-dried using procedure 1 |
Serum freeze-dried using procedure 2 |
|
Appearance |
Dry homogeneous amorphous mass in the form of cake |
– |
+ |
|
Colour |
Light cream |
+ |
+ |
|
Extraneous matter, mold |
Not allowed |
+ |
+ |
|
Moisture content, %, maximum |
4 |
+ |
+ |
|
Solubility |
The contents of the ampoules shall dissolve in saline solution within 2–5 minutes and be a homogeneous suspension |
– (undissolved fragments are observed after 10 minutes) |
+ |
|
Activity: CFT titre, at least |
1:80 |
+ |
+ |
|
Specificity in CFT |
Shall react only with specific Chlamydia antigen |
+ |
+ |
It was found that the serum freeze-dried using procedure 1 did not comply with the specified characteristics. No cake-like homogeneous mass formed in the ampoules. The resulting serum did not dissolve properly in the water. The serum freeze-dried using procedure 2 fully complied with the specified characteristics.
The serum samples freeze-dried using procedure 2 were used for subsequent tests of hyperimmune serum for their activity during the long-term storage.
The results of tests of the freeze-dried Chlamydia hyperimmune serum for its antigenic activity carried out during 24 months after its freeze-drying are shown in Figure 4.
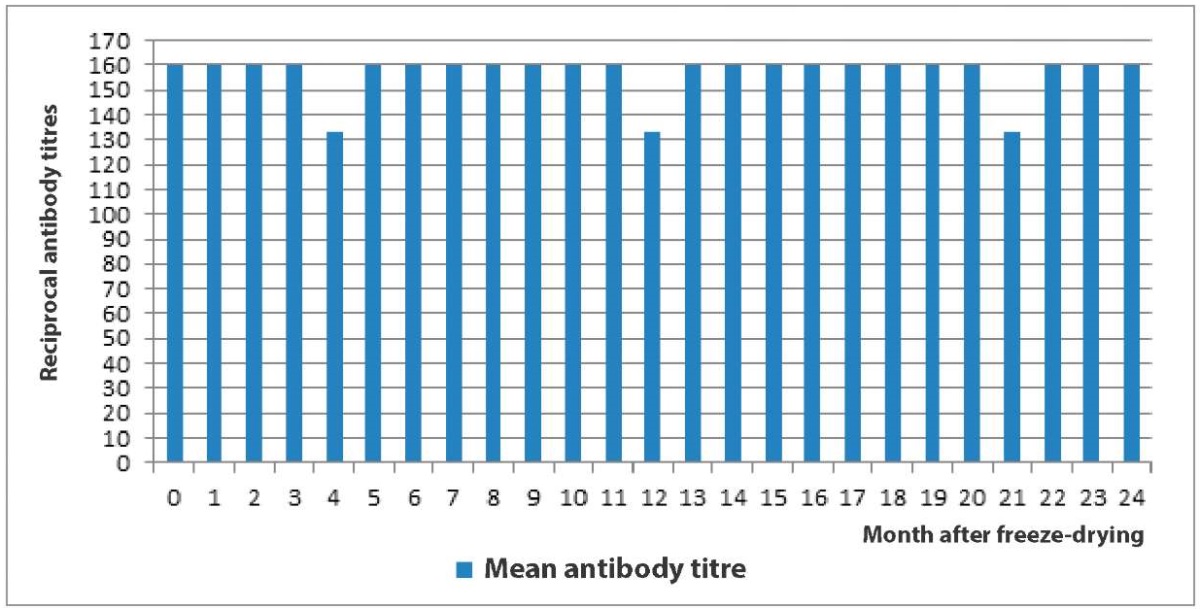
Fig. 4. Anti-Chlamydia hyperimmune ovine serum activity during its long-term storage (24 months)
It was found that the activity of hyperimmune sheep sera did not decrease below the antibody titre specified in the technical specifications (1:40) during two years after its freeze-drying. On month 4, 12 and 21 of storage, one serum out of three reacted at a titre of 1:80, so the mean titre at this time of the study was 1:133. During the following months, all sera reacted at a titre of 1:160. A decrease in the titer of anti-Chlamydia antibodies detected at some time points of the study could be associated with errors in CFT procedure.
DISCUSSION
As practice shows freeze-drying is the optimal preservation method for the sera intended for long-term storage [20]. Previously, optimal freeze-drying procedure was determined for the equipment available at that time for freeze-drying of the specific anti-Chlamydia serum being a component of the manufactured diagnostic kit. The sera freeze-dried using that procedure complied with the technical specifications for the manufactured test-kit. New modern freeze-dryer with more powerful characteristics, larger drying chamber and electronic control unit enabling temperature and vacuum monitoring and regulating, was purchased as part of technical renovation of the production site.
Two procedures for specific anti-Chlamydia serum freeze-drying are described in the paper. The first procedure was as close as possible to the freeze-drying procedure used for old equipment. That freeze-drying procedure was found unsuitable for the new equipment. Therewith, a new freeze-drying procedures was developed (the second procedure).
Information on the development of freeze-drying procedures for specific sera and immunoglobulins used for diagnostic test systems is available in the scientific literature. The freeze-drying period for these products ranges from 24 to 30 hours [14][16][21][22]. The first freeze-drying procedure used during the study took 26 hours. The freeze-drying procedure developed for the new equipment (the second freeze-drying procedure) made it possible to reduce the drying time to 22 hours, which was the first difference between the two methods.
The second difference was the temperature conditions for heating of freeze-dryer shelves. With the first procedure for the specific serum freeze-drying, the temperature of the freeze-dryer shelves ranged from minus 39 to minus 38 °C for the first 6 hours. Whereas with the second procedure for specific serum freeze-drying, the serum was loaded into freeze-dryer chamber cooled to minus 35 °C and starting from the first hour the shelves were heated first to minus 4 °C (for the first 8 hours), then to plus 0.5 °C (for the next 10 hours). The sublimation process at the specified parameters in the first case lasted for 22 hours, in the second case – for 20 hours. In the first case, the sublimation process was too slow during the first 6 hours after the freeze-drying start due to very low temperature and relatively low vacuum. Then, during heating of the shelves, the vacuum levels throughout the drying process also remained low (from 135 to 101 Pa at the sublimation stage). All of this together prevented complete moisture evaporation from the serum during set time period. The residual moisture condensed during the final drying process and dissolved part of the freeze-dried serum cake in the ampoule (Fig. 3). The optimal temperature and vacuum enabling preparation of freeze-dried specific serum of appropriate quality were selected for the second freeze-drying procedure.
In the paper of A. V. Komissarov et al. data on the correlation of the serum freeze-drying time with the amount of the serum to be freeze-dried are presented. The data indicate that the freeze-drying time reduces with a decrease in the amount of the serum loaded into the freeze-dryer [14]. In our study, not all moisture evaporated and condensed on serpentine cooler during the set time interval when the first freeze-drying procedure was used, although this freeze-drying procedure was optimal for the previous freeze-dryer. This was accounted for increase in the amount of the serum subjected to freeze-drying in the new freeze-dryer having larger drying chamber. Considering this, in order to optimize the freeze-drying process, the time for cooling freeze-dryer shelves could be increased, which would improve the drying process, but would significantly extend the sublimation time period. However, the period of freeze-drying of various products can be reduced by selecting the optimal mode of accelerated heating of the freeze-dryer shelves within subzero temperatures at the stage of sublimation [23]. This principle was used for the second procedure for specific anti-Chlamydia serum freeze-drying. The new procedure for serum freeze-drying has enabled preparation of a high-quality component for the diagnostic kit in large volumes.
CONCLUSION
Freeze-drying process for specific anti-Chlamydia serum intended for the diagnostic test kit has been optimized during the study. The resulting serum is fully compliant with characteristics laid down in the technical specifications for the diagnosticum, TU 9388-020-00492374-2007 “Antigen and Serum Kit for Serological Diagnosis of Chlamydiosis in Livestock”.
The optimized freeze-dried procedure differed from the previously used procedure in the reduced heating time of the freeze-dryer shelves at subzero temperatures and increased vacuum, which, in turn, enabled shortening of the serum freeze-drying time and preparation of high-quality component used for the test-kit manufacturing.
It has been established that the freeze-dried serum shelf life is at least two years, during this period the serum retains its activity and physico-chemical properties.
References
1. Feodorova V. A., Lyapina A. M., Khizhnyakova M. A., Zaitsev S. S., Saltykov Yu. V., Subbotina I. A., et al. Chlamydioses in animals and humans. Moscow: Nauka; 2019. 135 p. https://doi.org/10.7868/9785020402492 (in Russ.)
2. Ravilov R. Kh. Kliniko-ehpizootologicheskie osobennosti khlamidioza u sel’skokhozyaistvennykh zhivotnykh = Clinical and epizootiological features of chlamydiosis in livestock. Proceedings of the VIEV. 2021; 82 (1): 174–177. https://elibrary.ru/qmaazo (in Russ.)
3. Krasochko P. A., Maksimovich V. V., Sinitsa N. V., Krasochko P. P., Konotop D. S., Yaromchik Ya. P., et al. Chlamydiosis in livestock: a study guide. Vitebsk: Vitebsk the Order of “the Badge of Honor” State Academy of Veterinary Medicine; 2020. 44 p. https://repo.vsavm.by/handle/123456789/14804 (in Russ.)
4. Cheong H. C., Lee C. Y. Q., Cheok Y. Y., Tan G. M. Y., Looi C. Y., Wong W. F. Chlamydiaceae: diseases in primary hosts and zoonosis. Microorganisms. 2019; 7 (5):146. https://doi.org/10.3390/microorganisms7050146
5. Mustafayeva N. A., Safarova S. A., Dzhumshudova F. A., Babanly L. T., Mammadova M. A. Chlamidiosis of agricultural animals. Prikaspijskij vestnik veterinarii. 2023; (1): 24–28. https://elibrary.ru/lvvhsy (in Russ.)
6. Borel N., Sachse K. Zoonotic transmission of Chlamydia spp.: Known for 140 years, but still underestimated. In: Zoonoses: Infections Affecting Humans and Animals. Еd. by A. Sing. Cham: Springer; 2023; 1–28. https://doi.org/10.1007/978-3-030-85877-3_53-1
7. Caspe S. G., HillH. Chlamydiosisin animals. Animals. 2024; 14 (21):3130. https://doi.org/10.3390/ani14213130
8. Ravilov A. Z., Gaffarov H. Z., Ravilov R. H. Chlamydiosis in animals. Kazan: Feng Publishing House of the Academy of Sciences of the Republic of Tatarstan; 2004. 368 p. (in Russ.)
9. Marti H., Jelocnik M. Animal Chlamydiae: A concern for human and veterinary medicine. Pathogens. 2022; 11 (3):364. https://doi.org/10.3390/pathogens11030364
10. Evstifeev V. V., Khusainov F. M., Khusainova G. I., Akbashev I. R., Yakovlev S. I. Otsenka effektivnosti razlichnykh spetsificheskikh khlamidiinykh antigenov dlya serologicheskoi diagnostiki = Evaluation of various specific Chlamydia antigens intended for serological diagnosis for their effectiveness. Innovatsionnye resheniya aktual’nykh voprosov bezopasnosti: sbornik materialov mezhdunarodnoi nauchno-prakticheskoi konferentsii (Kazan, 11–12 noyabrya 2021 g.) = Innovative solutionsto topicalsafety issues: proceedings of the International Research-to-Practice Conference (Kazan, 11–12 November 2021). Kazan: Federal Center for Toxicological, Radiation and Biological Safety; 2021; 47–51. https://elibrary.ru/ikekgw (in Russ.)
11. Evstifeev V. V., Khusainov F. M., Khusainova G. I., Akbashev I. R., Yakovlev S. I., Khamidullina R. Z. Activity and specificity of lyophilized antigens and sera for serological diagnosis of Chlamydia. Molodezhnaya nauka – razvitiyu agropromyshlennogo kompleksa: materialy Vserossiiskoi (natsional’noi) nauchno-prakticheskoi konferentsii studentov, aspirantov i molodykh uchenykh (Kursk, 3–4 dekabrya 2020 g.). Chast’ 2 = Early career researchers – for the development of agroindustry: proceedings of the All-Russia (National) Research-to-Practice Conference of Students, Postgraduate Students and Early Career Researchers (Kursk, 3–4 December 2020). Part 2. Kursk: Kursk State Agricultural Academy; 2020; 476–480. https://elibrary.ru/psgqhb (in Russ.)
12. Zhdanova E. V., Rusanova D. V., Semircheva A. A., Geogdjayan A. S., Zharnikova I. V. Kontrol’ stabil’nosti diagnostikuma eritrotsitarnogo brutselleznogo antigennogo zhidkogo v protsesse khraneniya = Storage stability tests of liquid erythrocyte brucellosis diagnosticum. Infektsionnye bolezni v sovremennom mire: evolyutsiya, tekushchie i budushchie ugrozy: materialy XIII Ezhegodnogo vserossiiskogo kongressa po infektsionnym boleznyam imeni akademika V. I. Pokrovskogo (Moskva, 24–26 maya 2021 g.) = Infectious diseases in the world today: evolution, current and future threats: proceedings of the ХIII Annual All-Russia Congress on Infectious Diseases named after Academician V. I. Pokrovsky (Moscow, 24–26 May 2021). Moscow: Meditsinskoe marketingovoe agentstvo; 2021; 189. https://elibrary.ru/kxkaxj (in Russ.)
13. Komissarov A. V., Bibikov D. N., Badarin S. A., Sinitsyna N. V., KostylevaN. I., Ovchinnikova M. V., et al. Calculation of dependencesfor estimating the amount of weight loss during lyophilization of diagnostic preparations. Proceedings of Universities. Applied Chemistry and Biotechnology. 2020; 10 (3): 506–514. https://doi.org/10.21285/2227-2925-2020-10-3-506-514 (in Russ.)
14. Komissarov A. V., Badarin S. A., Bibikov D. N., Sinitsyna N. V., Kostyleva N. I., Glazkova E. A., et al. Improvement freeze-drying of diagnostic cholera sera in ampoules. Bacteriology. 2023; 8 (2): 34–41. https://elibrary.ru/hzodwc (in Russ.)
15. Nevskaia L. V., Voropaev A. A., FadeikinaO. V., Petrova N. E., KapitanovaV. K., TregubovaV. E., et al. Method of obtaining lyophilized form ofserum reference sample containing allergen-specific immunoglobulin E (versions). Patent No. 2802333 C1 Russian Federation, Int. Cl. А61К 39/395 (2006.01), А61К 9/19 (2006.01), А61К 38/00 (2006.01). Scientific Centre for Expert Evaluation of Medicinal Products’ of the Ministry of Health of the Russian Federation. No. 2022132754. Date of filing: 14.12.2022. Date of publication: 24.08.2023. Bull. No. 24. (in Russ.)
16. Ivanova S. V., Melnikova L. A., Rodionov A. P., Evstifeev V. V. Method of obtaining and storing hyperimmune anthrax serum. Veterinary Science Today. 2023; 12 (3): 215–221. https://doi.org/10.29326/2304-196X-2023-12-3-215-221
17. Yakovlev S. I. Improvement of tools for specific prevention of chlamydiosis in animals: Author’s thesis for degree of Cand. Sci. (Veterinary Medicine). Moscow; 2022; 87–88. (in Russ.)
18. Evstifeev V. V. Development and improvement of biologicals for diagnosis and specific prevention of chlamydiosisin animals: Author’sthesis for degree of Dr. Sci. (Biology). Kazan; 2015. 417 p. (in Russ.)
19. GOST 24061-2012 Medicine remedies biological lyophilized for veterinary use. Method for determination mass moisture. https://docs.cntd.ru/document/1200103299 (in Russ.)
20. Fissore D., McCoy T. Editorial: Freeze-drying and process analytical technology for pharmaceuticals. Frontiers in Chemistry. 2018; 6:622. https://doi.org/10.3389/fchem.2018.00622
21. Komissarov A. V., Ovchinnikova M. V., Badarin S. A., Bibikov D. N., Sinitsyna N. V., Kostyleva N. I., Plotnikov I. A. Experimental substantiation of new presentation form of cholera diagnostic sera. Problems of Particularly DangerousInfections. 2017; (4): 38–40. https://doi.org/10.21055/0370-1069-2017-4-38-40 (in Russ.)
22. Kochkalova N. N., Abramova E. G., Nikiforov A. K., Kireyev M. N., Lobovikova O. A., Savitskaya L. V., et al. Optimization of presentation and consumer container of anti-rabies immunoglobulin obtained from horse serum. Bulletin ofthe East Siberian ScientificCenter oftheAcademy ofMedical Sciences. 2012; (5-1): 236–238. https://elibrary.ru/piwdvz (in Russ.)
23. Alekseev K. V., Blynskaya E. V., Tishkov S. V. Theory and practice of medicinal product freeze-drying: a monograph. Moscow: Printing House “Mittel Press”; 2019. 219 p.
About the Authors
V. V. EvstifeevRussian Federation
Vitaliy V. Evstifeev, Dr. Sci. (Biology), Associate Professor, Chief Researcher, Laboratory for Viral Anthropozoonoses of Animals
Nauchnyi gorodok-2, Kazan 420075, Republic of Tatarstan; 35 Sibirskiy trakt str., Kazan 420029, Republic of Tatarstan
S. I. Yakovlev
Russian Federation
Sergey I. Yakovlev, Cand. Sci. (Veterinary Medicine), Researcher, Laboratory for Viral Anthropozoonoses of Animals
Nauchnyi gorodok-2, Kazan 420075, Republic of Tatarstan
F. M. Khusainov
Russian Federation
Fidail M. Khusainov, Dr. Sci. ( Veterinary Medicine), Associate Professor, Leading Researcher, Laboratory for Viral Anthropozoonoses of Animals
Nauchnyi gorodok-2, Kazan 420075, Republic of Tatarstan
I. R. Akbashev
Russian Federation
Ilgizar R. Akbashev, Cand. Sci. (Veterinary Medicine), Researcher, Laboratory for Animal Viral Diseases
Nauchnyi gorodok-2, Kazan 420075, Republic of Tatarstan
S. V. Sadykova
Russian Federation
Svetlana V. Sadykova, Junior Researcher, Laboratory for Animal Viral Diseases
Nauchnyi gorodok-2, Kazan 420075, Republic of Tatarstan
Review
For citations:
Evstifeev V.V., Yakovlev S.I., Khusainov F.M., Akbashev I.R., Sadykova S.V. Optimization of freeze-drying process for anti-Chlamydia hyperimmune serum. Veterinary Science Today. 2025;14(1):82-89. https://doi.org/10.29326/2304-196X-2025-14-1-82-89



































