Scroll to:
Extension of scope of susceptible mammalian species as avian influenza global situation developed in 2023–2024
https://doi.org/10.29326/2304-196X-2025-14-1-6-13
Abstract
Introduction. Highly pathogenic avian influenza currently requires the close attention of the international community. Determining the factors affecting transmission and replication of avian influenza virus in mammals and analysing the evolutionary processes involved will suggest which virus lineages will have the spillover potential and infect non-typical hosts, including humans.
Objective. The paper is aimed at studying the avian influenza epidemic situation in mammals, description of the features of the avian influenza epizootic process, retrospective analysis of influenza outbreaks in non-typical hosts.
Materials and methods. The study was carried out in the Information and Analysis Centre of the Veterinary Surveillance Department of the Federal Centre for Animal Health (Vladimir). The data obtained was based on statistical data from the database of the World Organisation for Animal Health WAHIS and scientific publications of foreign and domestic authors. Cartographic analysis was carried out using ArcGIS geographic information system (ESRI, USA).
Results. The avian influenza virus H5N1 epizootic process in 2022–2024 involved mammalians of various families (Bovidae, Mustelidae, Ursidae etc.) in which the disease had not been previously recorded. Strict biosecurity measures and updated alert systems are of crucial importance to effectively prevent the spread of the disease. In a limited number of countries (Bangladesh, Dominican Republic, China, Egypt, Indonesia, Laos, Vietnam, EU countries, etc.), vaccination has been used as a preventive and emergency measure to protect birds from influenza.
Conclusion. Transmission of highly pathogenic avian influenza virus to mammals of different species, including livestock, may be the start of a future pandemic. The recently recorded virus spillover indicates emergence of adaptive mutations and poses a threat to animal health, public health, food security and biodiversity.
Keywords
For citations:
Zhiltsova M.V., Akimova T.P., Mitrofanova M.N., Semakina V.P., Vystavkina E.S. Extension of scope of susceptible mammalian species as avian influenza global situation developed in 2023–2024. Veterinary Science Today. 2025;14(1):6-13. https://doi.org/10.29326/2304-196X-2025-14-1-6-13
INTRODUCTION
Avian influenza virus continues to pose a threat to animal and human health. H5 and H7 subtypes have caused numerous outbreaks in wild and domestic birds and resulted in mortality of at least 600 million poultry since 2005. Many countries are now concerned about the development and application of different strategies aimed at avian influenza control.
Unlike H5N2, H5N3, H5N4, H5N5 and H5N6 subtypes detected in a rather limited area or within the continent, the H5N1 subtype started a large-scale intercontinental spread [1].
H5N1 subtype has caused a significant number of outbreaks in many countries in Europe, Africa, Asia and the Americas [2].
The virus interspecies spillover usually results in a dead-end infection. The probability that a complete set of adaptive mutations is acquired in a single immunocompetent host and transmitted onwards to other hosts is extremely low. Adaptive mutations occurring during an epizooty could enhance adaptiveness of the virus through increased polymerase activity to allow transmission to less susceptible hosts. This is demonstrated by the results of both experimental infections and isolation of the virus from atypical wild and livestock hosts during outbreaks: spread of high pathogenicity avian influenza (HPAI) H5N1 to farmed pigs in Indonesia, and transmission to cattle and goats in the USA [3].
Evident changes in the epidemiology and ecology of the virus now pose a threat to animal health, public health, food security and biodiversity. Conventional control measures such as biosecurity, stamping-out and movement restrictions, although important, may not be sufficient. Most countries have mechanisms in place to facilitate the regular exchange of information and best practices to coordinate disease control policies and develop evidence-based national strategies [4][5].
The aim of the study was to investigate the avian influenza epizootic situation in mammals, to characterize the avian influenza epizootic process and to retrospectively analyze influenza outbreaks in atypical hosts.
MATERIALS AND METHODS
The study was carried out in the Information Analysis Centre of the Veterinary Surveillance Department, Federal Centre for Animal Health (Vladimir). The data were collected using statistical material from the WAHIS database of the World Organisation for Animal Health (WOAH) and scientific publications of foreign and domestic authors. Cartographic analysis was carried out using ArcGIS geographic information system (ESRI, USA).
DYNAMICS OF HPAI OUTBREAKS AMONG ATYPICAL HOSTS, INCLUDING LIVESTOCK
During the ongoing global outbreak of avian influenza caused by the HPAI A/H5N1 virus, both birds and many mammalian species were found to be infected. In 2022–2024 there was a marked change in the scope and ratio of atypical hosts naturally infected with HPAI. Mammalian species, including cattle and small ruminants, were involved in the process.
The significant increase in the number of detected mammalian infections (Fig. 1) from 139 in 2022 to 275 in 2023 is linked to the spread of infection and implementation of expanded avian influenza monitoring programmes [6]. It cannot be excluded that some atypical hosts may be important reservoirs of infection. HPAI A/H5N1 virus has recently demonstrated easy spillover into wildlife and agriculture, and has the potential to trigger a global pandemic.
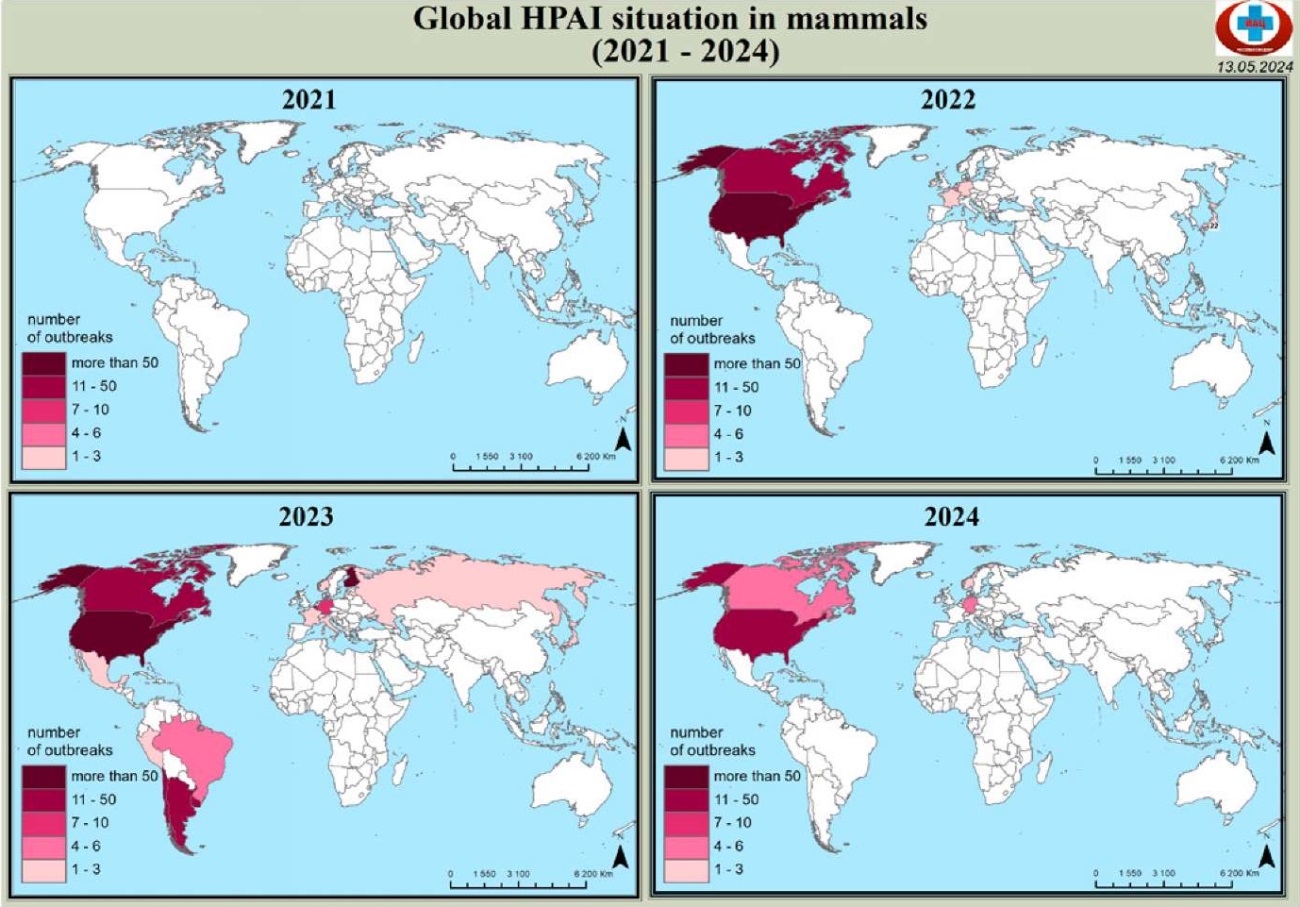
Fig. 1. Avian influenza epizootic situation in mammals in 2021–2024
The virus has currently impacted a variety of mammalian species worldwide, including those classified as endangered and threatened, potentially exacerbating their conservation status. The most likely source of mammalian infection appears to be close contact with infected birds, with some evidence suggesting potential mammal-to-mammal transmission [7].
Previously we described the HPAI global situation in mammals in 2022 [8]. The virus was said to have a high ability to spillover from birds to mammals such as mustelids (minks, otters, ferrets, badgers), felines (domestic cats, cougars, leopards, lynxes), pinnipeds (common seals, grey seals), bears (brown, grizzly, American black), bottlenose dolphins, skunks, foxes, opossums, raccoons. HPAI manifestations in mammals range from asymptomatic to severe forms.
HPAI virus strains that have already adapted to various mammalian species currently continue to circulate.
HPAI EPIZOOTIC SITUATION IN ATYPICAL HOSTS IN 2023–2024
In addition to the increased number of HPAI cases reported in mammals, there has been a change in the ratio of diseased animals belonging to various families over the last 2 years (Fig. 2, Table).
Thus, in 2022 more than 50% of infected animals belonged to the Canidae family (red foxes). In 2023, there was an increase in the number of cases among pinnipeds, felines, farmed mustelids, as well as among new species: forest polecat, coatis. In 2024 a significant number of outbreaks were recorded in bovids (cattle, goats).
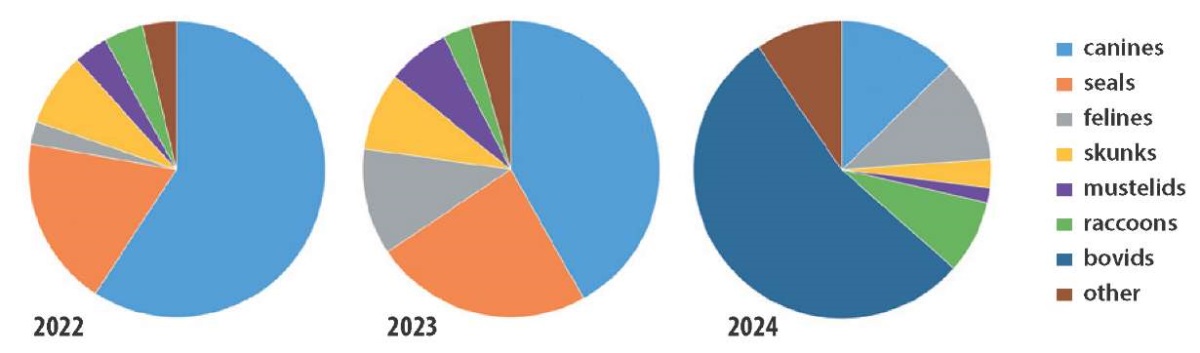
Fig. 2. Global distribution of avian influenza outbreaks by mammalian families in 2022–2024
Table
Highly pathogenic avian influenza H5 in mammals in 2023–2024 (according to the WOAH data)
|
Country |
Animal species |
Outbreaks |
Virus type |
|
Argentina |
South American fur seal |
2 |
H5 |
|
South American sea lion |
14 |
H5 |
|
|
Southern elephant seal |
2 |
H5 |
|
|
Belgium |
Red fox |
16 |
not typed |
|
Forest polecat |
2 |
not typed |
|
|
Brazil |
South American fur seal |
5 |
H5N1 |
|
South American sea lion |
|||
|
Germany |
Grey seal |
1 |
H5N1 |
|
Pine marten |
1 |
H5N1 |
|
|
Red fox |
6 |
H5N1 |
|
|
Raccoon |
1 |
H5N1 |
|
|
Italy |
Domestic cat |
1 |
H5N1 |
|
Domestic dog |
1 |
H5N1 |
|
|
Red fox |
2 |
H5N1 |
|
|
Canada |
American mink |
2 |
H5N1 |
|
Raccoon |
3 |
H5N1 |
|
|
Raccoon |
3 |
H5N5 |
|
|
Domestic cat |
2 |
H5N1 |
|
|
Red fox |
1 |
H5N5 |
|
|
Red fox |
7 |
H5N1 |
|
|
Skunk |
1 |
H5N5 |
|
|
Skunk |
9 |
H5N1 |
|
|
Domestic dog |
1 |
H5N1 |
|
|
Latvia |
Red fox |
1 |
H5N1 |
|
Norway |
Red fox |
1 |
H5N1 |
|
Red fox |
2 |
H5N5 |
|
|
Peru |
South American sea lion |
2 |
H5 |
|
Lion |
1 |
H5 |
|
|
Russia |
Northern fur seal |
1 |
H5N1 |
|
USA |
American mink |
2 |
H5N1 |
|
American black bear |
1 |
H5N1 |
|
|
USA |
Abert’s squirrel |
1 |
H5N1 |
|
Polar bear |
1 |
H5N1 |
|
|
Dolphin [13] |
1 |
H5N1 |
|
|
Raccoon |
6 |
H5N1 |
|
|
Fisher |
2 |
H5N1 |
|
|
Domestic cat |
11 |
H5N1 |
|
|
Common seal |
1 |
H5N1 |
|
|
Opossum |
1 |
H5N1 |
|
|
Cougar |
17 |
H5N1 |
|
|
Red fox |
20 |
H5N1 |
|
|
Lynx |
6 |
H5N1 |
|
|
Skunk |
17 |
H5N1 |
|
|
Goat |
1 |
H5N1 |
|
|
Cattle |
33 |
H5N1 |
|
|
Uruguay |
Coatis |
1 |
H5N1 |
|
South American sea lion |
8 |
H5N1 |
|
|
South American fur seal |
3 |
H5N1 |
|
|
Finland |
American mink |
6 |
H5N1 |
|
Otter |
2 |
H5N1 |
|
|
Raccoon dog |
9 |
H5N1 |
|
|
Red fox |
13 |
H5N1 |
|
|
Arctic fox |
48 |
H5N1 |
|
|
Lynx |
1 |
H5N1 |
|
|
Sable |
1 |
H5N1 |
|
|
France |
Red fox |
1 |
H5N1 |
|
Chile |
Marine otter |
2 |
H5 |
|
Eurasian river otter |
1 |
H5 |
|
|
South American sea lion |
31 |
H5 |
|
|
South Korea |
Domestic cat |
2 |
H5N1 |
|
Japan |
Red fox |
2 |
H5N1 |
Deaths of seals due to HPAI A/H5N1 infection in 2022 have been confirmed in Quebec (Canada) and along the US coast [9]. HPAI outbreak that began in November 2022 in Peruvian pelicans along the Peruvian coast and adjacent islands spread to marine mammals, particularly to South American sea lions, causing mass mortality by early 2023. Researchers have confirmed the virus entry into Peru from North America, presumably due to wild bird migration [10][11][12][13].
There have been several cases of HPAI H5 transmission among other domestic and wild birds, as well as zoo animals and wild predators.
Extension of the species range and increase in the number of reported cases are not only linked to the disease spread, but also to the implementation of HPAI monitoring programmes in various countries [6].
In 2024, infection cases of influenza A/H5N1 began to be reported in cattle. Reports coming from the USA on HPAI positive tests in dairy cattle and goats, as well as probable transmission of the H5N1 subtype virus between cattle in dairy herds, are of concern because of the possibility of rapid adaptation and further interspecies spillover. All animals demonstrated similar clinical signs [14][15]. The likely source of infection was cow feed or water that were accessible to wild birds. Cases of HPAI transmission from cattle to humans were recorded [16][17].
Recombination of the North American viruses probably occurred shortly before the outbreak emerged in cattle. All isolates recovered from cattle were reassortants of the Eurasian and North American genotypes first detected in late 2023. The outbreak in goats was not linked to the outbreak in cattle. The HPAI H5N1 outbreak in cattle likely went undetected for an extended period. Researchers assume that the onset of the event occurred between 13 November 2023 and 18 January 2024 [18].
In light of recent HPAI situation in the USA the American Association of Bovine Practitioners (AABP) has taken steps to redefine the disease syndrome caused by the avian influenza virus and observed in cattle and designate it as bovine influenza A virus (BIAV), which requires further studies [19][20][21].
Experts at the Centers for Disease Control and Prevention (CDC) believe that the current HPAI-associated risk to humans is low, but people who come in contact with infected birds or animals are at greater risk of contracting HPAI A/H5N1 [22].
Infected cattle showed a non-specific course of infection, reduced feed intake and a sharp drop in milk yield, but severe systemic influenza infection was demonstrated in domestic cats receiving raw (unpasteurised) milk from diseased cows. In addition, cases of cow-to-cow transmission were reported [23].
In response to this situation, the GF-TADs (Global Framework for the Progressive Control of Transboundary Animal Diseases) meeting was held on 4 April 2024 to address identified cases of high pathogenicity influenza in dairy cattle and goats in the United States of America and the detection of the virus in humans. The importance of early detection and transparency of notifications, as well as cooperation between different national agencies, was emphasized [24].
The WOAH continues to monitor the situation to determine the risks to animal and human health. Timely reporting is crucial to objectively assess the disease situation and prevent any type of misinformation. Based on the data available the WOAH points out that restrictions on the movement of healthy cattle and products thereof are not recommended unless justified by an import risk analysis conducted in accordance with Chapter 2.1 of the WOAH Terrestrial Animal Health Code [25][26].
GLOBAL CONTROL STRATEGIES
The first protective measure against HPAI spread is early detection of outbreaks. Establishing accurate warning systems is essential for effective prevention and control of the disease. Strict biosecurity and hygiene measures are also necessary to prevent outbreaks. When infection is detected in poultry, a culling policy is usually applied [27].
Vaccination of poultry may be recommended under certain conditions. Vaccines used should comply with the standards specified in the WOAH guidelines [28]. In early 2023 it was allowed to conduct emergency vaccination of wild birds against HPAI as an immediate response to an outbreak or when there was an increased risk of infection entry.
Concerns about international trade restrictions hamper use of vaccination, although its inclusion as a control tool has been endorsed by international standards adopted by the World Assembly of WOAH National Delegates. Unjustified trade restrictions on poultry and poultry products from vaccinated flocks have a huge impact on a sector that contributes significantly to global food security and the economy [29].
To date, vaccination has only been used in a limited number of countries as a preventive or emergency measure to protect birds against HPAI [30][31]. According to various sources (including the WOAH), more than 30 countries have resorted to the use of vaccination against avian influenza since 2005 [32][33][34]. The countries that have officially declared HPAI vaccination are: Armenia, Belarus, Bangladesh, Dominican Republic, China (including Hong Kong), Egypt, El Salvador, Germany, Indonesia, Jordan, Kazakhstan, Democratic People’s Republic of Korea, Kuwait, Laos, Mongolia, Mexico, Niger, Pakistan, Peru, Singapore, Sudan, Turkmenistan, Vietnam, Ecuador, Uruguay and others. In some European countries (Ireland, Great Britain) vaccination is allowed only in zoos [35][36][37]. In Russia, preventive vaccination against HPAI is practiced in farms (except poultry farms) according to the “Veterinary rules for the implementation of preventive, diagnostic, restrictive and other measures, establishment and lifting of quarantine and other restrictions aimed at preventing spread and eradication of high pathogenicity avian influenza”, approved by Order of the Ministry of Agriculture of Russia No. 158 of 24 March 2021.
In May 2023 the US Animal and Plant Health Inspection Service (APHIS) announced that it had approved the emergency use of avian influenza vaccine to prevent additional deaths among California condors. Prior to this outbreak, the US authorities had stated that strict biosecurity protocols, including enhanced disinfection procedures as well as destruction of infected birds, were sufficient to mitigate the HPAI effects. Work is currently underway to develop a vaccine against HPAI for cattle, as it is believed that vaccination will help reduce the risk of the disease spreading to new animal species and lower potential losses to dairy production facilities. Vaccination of farm poultry has long been controversial among researchers and farmers in the US. Poultry producers are concerned about the cost and complexity of vaccinating millions of birds, as well as trade restrictions [38][39].
In May 2023, the 27 Member states of the European Union agreed to implement a vaccination strategy against avian influenza. The tasks were shared between the states: France developed a vaccine for ducks, the Netherlands – for laying hens, Italy – for turkeys and Hungary – for Peking ducks. Preliminary results were promising: in Hungary, the mortality of vaccinated geese (HVT-H5 manufactured by Ceva Sante Animale) after challenge was 2.93% compared to 76.23% in the control group, and there was also a reduction in the viral shedding. In Italy, a high level of clinical protection of turkeys was achieved using HVT-H5 vaccine with a subunit or DNA vaccine administered as a booster. Homologous vaccination gave unsatisfactory results (25 to 40% protection) [40].
Study results of Dutch researchers showed that both HVT-H5 vaccines by Ceva Animal Health and Boehringer Ingelheim effectively protected poultry in 8 weeks after vaccination [41].
France was the first European country to introduce mandatory vaccination of ducks from October 2023, despite the risk of trade restrictions introduced by third countries (USA, Japan) [42][43][44]. No outbreaks were reported among vaccinated poultry in the south of the country thereafter. In the period from 2 December 2023 to 15 March 2024 the diseased birds were predominantly found in unvaccinated poultry populations. The number of HPAI cases detected in wild birds and commercial poultry was lower than in the same period last year [45]. According to the European Food Safety Authority report, no outbreaks were reported in poultry in France from 16 March to 14 June 2024 [46].
Positive results of HPAI vaccination are also recorded in Bangladesh, where poultry have been vaccinated since 2012. The number of outbreaks before vaccination was 18 times higher than after it. The latest avian influenza outbreaks in the country were reported in 2019. The studies conducted in Bangladesh suggest that poultry vaccination can be part of a holistic strategy to mitigate HPAI consequences if accompanied by monitoring to avoid latent spread [47][48].
Preventive vaccination has also been used successfully in Hong Kong since 2003, where no HPAI outbreaks in poultry population have been reported since 2018 [49].
Emergency vaccination with Nobilis Influenza H5N2 vaccine conducted in the Czech Republic in 2021 helped to preserve the national breed of geese. Poultry breeders and the public perceive the possibility of vaccination very positively [40].
CONCLUSION
Influenza A virus, including the H5N1 subtype, can infect many animal species. In recent years, some HPAI virus strains have adapted to new mammalian species, which demonstrates likelihood that the virus will acquire a set of additional adaptive mutations. Transmission of avian influenza A virus to mammals, including humans, could be the first step towards a future pandemic. Identification of factors affecting transmission and replication of the virus in mammals will make it possible to predict which viral lineages are more likely to spillover and cause disease in atypical hosts, including humans [50].
Although infection with HPAI mammalian strains is rare, a growing number of publications indicate an increasing prevalence of the disease, emphasizing the need for preventive measures to limit transmission, thereby preventing a potential future epidemic in humans.
Environmental and epizootological changes that occurred due to avian influenza outbreaks in recent years have raised doubts regarding the exclusive use of stamping-out programmes. International organisations (WOAH, European Food Safety Authority, European Commission) suggest that preventive vaccination can minimise the number of outbreaks and the duration of the epizootic. The use of vaccines may reduce the risk of avian influenza spillover to new animal species and lower potential losses. However, vaccination should complement, not replace, other preventive and control measures such as avian infection monitoring, early detection and biosecurity, and is recommended as part of a comprehensive approach to control of avian influenza outbreaks.
References
1. Food and Agriculture Organization of the United Nations. EMPRES Global Animal Disease Information System (EMPRES-i+). https://empres-i.apps.fao.org
2. WOAH. World Animal Health Information System (WAHIS). https://wahis.woah.org/#/home
3. Arruda B., Baker A. L. V., Buckley A., Anderson T. K., Torchetti M., Bergeson N. H., et al. Divergent pathogenesis and transmission of highly pathogenic avian influenza A(H5N1) in swine. Emerging Infectious Diseases. 2024; 30 (4): 738–751. https://doi.org/10.3201/eid3004.231141
4. WOAH. High pathogenicity avian influenza (HPAI) – situation report. https://www.woah.org/app/uploads/2024/04/hpai-situation-report-20240409.pdf
5. WOAH. Resolutions. Adopted by the World Assembly of Delegates During the 90th General Session. 21–25 May 2023. https://www.woah.org/app/uploads/2023/06/a-resos-2023-all.pdf
6. Shi J., Zeng X., Cui P., Yan C., Chen H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerging Microbes & Infections. 2023; 12 (1):2155072. https://doi.org/10.1080/22221751.2022.2155072
7. PoultryMed. Current trends in mammalian infection patterns with HPAIv H5N1. Emerging Infectious Diseases 2024. February 14, 2024. https://www.poultrymed.com/Poultrymed/Templates/showpage.asp?DBID=1&LNGID=1&TMID=178&FID=9025&PID=0&IID=89640
8. Zhiltsova M. V., Akimova T. P., Varkentin A. V., Mitrofanova M. N., Mazneva A. V., Semakina V. P., Vystavkina E. S. Global avian influenza situation (2019–2022). Host range expansion as evidence of high pathogenicity avian influenza virus evolution. Veterinary Science Today. 2023; 12 (4): 293–302. https://doi.org/10.29326/2304-196X-2023-12-4-293-302
9. Lair S., Quesnel L., Signore A. V., Delnatte P., Embury-Hyatt C., Nadeau M.-S., et al. Outbreak of highly pathogenic avian influenza A(H5N1) virus in seals, St. Lawrence Estuary, Quebec, Canada. Emerging Infectious Diseases. 2024; 30 (6): 1133–1143. https://doi.org/10.3201/eid3006.231033
10. Leguia M., Garcia-Glaessner A., Muñoz-Saavedra B., Juarez D., Barrera P., Calvo-Mac C., et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirdsin Peru. Nature Communications. 2023; 14:5489. https://doi.org/10.1038/s41467-023-41182-0
11. Servicio Nacional de Áreas Naturales Protegidas por el Estado. Sernanp despliega protocolo de monitoreo ante casos de aves y lobos marinos afectados por influenza aviar en áreas naturales protegidas. https://www.gob.pe/institucion/sernanp/noticias/697084-sernanp-despliega protocolo-de-monitoreo-ante-casos-de-aves-y-lobos-marinos-afectados-por-influenza-aviar-en-areas-naturales-protegidas (in Spanish)
12. Pardo-Roa C., Nelson M. I., Ariyama N., Aguayo C., Almonacid L. I., Munoz G., et al. Cross-species transmission and PB2 mammalian adaptations of highly pathogenic avian influenza A/H5N1 viruses in Chile. bioRxiv (Preprint). 2023; 2023.06.30.547205. https://pubmed.ncbi.nlm.nih.gov/37786724
13. Murawski A., Fabrizio T., Ossiboff R., Kackos C., Jeevan T., Jones J. C., et al. Highly pathogenic avian influenza A(H5N1) virus in a common bottlenose dolphin (Tursiops truncatus) in Florida. Communications Biology. 2024; 7:476. https://doi.org/10.1038/s42003-024-06173-x
14. Ly H. Highly pathogenic avian influenza H5N1 virus infections of dairy cattle and livestock handlersin theUnited States of America. Virulence. 2024; 15 (1):2343931. https://doi.org/10.1080/21505594.2024.2343931
15. Branda F., Romano C., Giovanetti M., Ciccozzi A., Ciccozzi M., Scarpa F. Emerging threats: Is highly pathogenic avian influenza AH5N1 in dairy herds a prelude to a new pandemic? Travel Medicine and Infectious Disease. 2024; 59:102721. https://doi.org/10.1016/j.tmaid.2024.102721
16. Cohen J. Worries about bird flu in U.S. cattle intensify. Science. 2024; 384 (6691): 12–13. https://doi.org/10.1126/science.adp6024
17. Polansek T., Steenhuysen J., Douglas L. Second US dairy worker infected with bird flu confirmed in Michigan. Reuters. May 23, 2024. https://www.reuters.com/world/us/second-human-case-bird-flu-linked-dairycows-detected-us-stat-news-reports-2024-05-22
18. PoultryMed. Genomic epidemiology of 2024 H5N1 outbreak in US cattle: Preliminary report. Infectious Diseases 2024. May 5, 2024. https://www.poultrymed.com/Poultrymed/Templates/showpage.asp?DBID=1&LNGID=1&TMID=178&FID=9025&PID=0&IID=89896
19. Brooks R. AABP decides to reference cattle disease as Bovine influenza A virus (BIAV). Bovine Veterinarian. April 9, 2024. https://www.bovinevetonline.com/news/industry/aabp-decides-reference-cattle-disease-bovine-influenza-virus-biav
20. McCormick L., Currin J. Bovine influenza A virus (BIAV) – HPAI in cattle. Virginia Cooperative Extension. April 23, 2024; APSC-197NP. https://ext.vt.edu/content/dam/pubs_ext_vt_edu/APSC/apsc-197/APSC-197.pdf
21. Bird flu strain found in US cows flown to UK lab for testing. Guardian. May 11, 2024. https://www.theguardian.com/world/article/2024/may/11/bird-flu-strain-found-in-us-cows-flown-to-uk-lab-for-testing
22. CDC. Highly Pathogenic Avian Influenza A(H5N1) Virus: Interim Recommendationsfor Prevention, Monitoring, and Public Health Investigations. Avian Influenza (Bird Flu). https://www.cdc.gov/bird-flu/prevention/hpai-interim-recommendations.html
23. Burrough E. R., Magstadt D. R., Petersen B., Timmermans S. J., Gauger P. C., Zhang J., et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerging Infectious Diseases. 2024; 30 (7): 1335–1343. https://doi.org/10.3201/eid3007.240508
24. WOAH. GF-TADs Meeting: Detection of HPAI in Ruminants and Humans in the USA. https://rr-americas.woah.org/en/news/gf-tads-meetingdetection-of-hpai-in-ruminants-and-humans-in-the-usa
25. WOAH. High Pathogenicity Avian Influenza in Cattle. https://www.woah.org/en/high-pathogenicity-avian-influenza-in-cattle
26. Import risk analysis. In: WOAH. Terrestrial Animal Health Code. 2024; Chapter 2.1. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/2023/chapitre_import_risk_analysis.pdf
27. WOAH. Avian Influenza. https://www.woah.org/en/disease/avianinfluenza/#ui-id-2
28. WOAH. Considerations for emergency vaccination of wild birds against high pathogenicity avian influenza in specific situations. 2023. https://www.woah.org/app/uploads/2024/01/vaccination-wild-birds-hpaioutbreak-dec2023.pdf
29. WOAH. Avian influenza vaccination: why it should not be a barrier to safe trade. https://www.woah.org/en/avian-influenza-vaccination-whyit-should-not-be-a-barrier-to-safe-trade
30. Byrne J. To what extentis vaccination against avian influenza increasing globally? FeedNavigator. News & Analysis on the Global Animal Feed and Pet Food Industries. February 14, 2024. https://www.feednavigator.com/Article/2024/02/14/Boehringer-talks-vaccination-campaigns-against-bird-flu
31. Bird flu vaccination policies by country. Reuters. February 17, 2023. https://www.reuters.com/business/healthcare-pharmaceuticals/bird-flu-vaccination-policies-by-country-2023-02-17
32. Swayne D. E., Sims L., Brown I., Harder T., Stegeman A., Abolnik C., et al. Technical Item. Strategic challenges in the global control of high pathogenicity avian influenza. 2023. https://www.woah.org/app/uploads/2023/05/a-90sg-8.pdf
33. Valencia A. Ecuador to vaccinate more than two million birds against bird flu. Reuters. February 1, 2023. https://www.reuters.com/world/americas/ecuador-vaccinate-more-than-two-million-birds-against-birdflu-2023-02-01
34. Cottrell E. Uruguay launches vaccination campaign to curb avian flu. WATTPoultry. May 9, 2023. https://www.wattagnet.com/egg/article/15537941/uruguay-launches-vaccination-campaign-to-curbavian-flu
35. WOAH. Self-declaration of the recovery of country freedom from infection with high pathogenicity avian influenza viruses (HPAI) by Ireland. https://www.woah.org/app/uploads/2022/12/2022-12-ireland-hpaiselfd.pdf
36. WOAH. Self-declaration of the recovery of a zone (Northern Ireland) free from infection with high pathogenicity avian influenza viruses (HPAI) in poultry by the United Kingdom. https://www.woah.org/app/uploads/2023/05/2023-03-northernirelandhpai-selfd-eng.pdf
37. WOAH. Self-declaration of the recovery of a zone (Great Britain) free from infection with high pathogenicity avian influenza viruses (HPAI) in poultry by the United Kingdom. https://www.woah.org/app/uploads/2024/05/2024-05uk-hpai-selfd-eng.pdf
38. Avian flu vaccine for California condors approved amid fears of extinction. Guardian. May 17, 2023. https://www.theguardian.com/usnews/2023/may/17/vaccine-california-condor-avian-influenza-near-extinction
39. Kozlov M. US will vaccinate birds against avian flu for first time – what researchers think. Nature. 2023; 618 (7964): 220–221. https://doi.org/10.1038/d41586-023-01760-0
40. Highly pathogenic avian influenza. Vaccination rules in the EU. WOAH 90thGeneral Session. https://food.ec.europa.eu/system/files/2023-06/ad_cm_ai_event-woah_20230522_pres_vaccination.pdf
41. Positieve resultaten testfase vaccineren tegen vogelgriepvirus. Rijksoverheid. May 28, 2024. https://www.rijksoverheid.nl/actueel/nieuws/2024/05/28/positieve-resultaten-testfase-vaccineren-tegen-vogelgriepvirus (in Dutch)
42. WOAH. Self-declaration by France on the recovery of freedom from highly pathogenic avian influenza in poultry. https://www.woah.org/app/uploads/2024/04/2024-03-france-hpai-selfd-eng.pdf
43. Influenza aviaire: le plan de vaccination de la France. https://agriculture.gouv.fr/tout-ce-quil-faut-savoir-sur-le-plan-daction-vaccinationiahp-en-france (in French)
44. Banoun H. Duck vaccination against bird flu in France. https://www. researchgate.net/publication/387723735_Duck_vaccination_against_bird_flu_in_France
45. European Food Safety Authority, European Centre for Disease Prevention and Control, European Union Reference Laboratory for Avian Influenza, Fusaro A., Gonzales J. L., Kuiken T., et al. Scientific report: Avian influenza overview December 2023 – March 2024. EFSA Journal. 2024; 22 (3):e8754. https://doi.org/10.2903/j.efsa.2024.8754
46. European Food Safety Authority, European Centre for Disease Prevention and Control, European Union Reference Laboratory for Avian Influenza, Alexakis L., Fusaro A., KuikenT., et al. Scientific report: Avian influenza overview March – June 2024. EFSA Journal. 2024; 22 (7):e8930. https://doi.org/10.2903/j.efsa.2024.8930
47. Islam Md. S., Islam Md. H., Sarder Md. J. U., Hossain K. M. M., Rahman Md. S., Akter M. R. Study on vaccination efficacy against avian influenza in Rajshahi, Bangladesh. Journal of Agricultural Science and Technology A. 2017; 7 (3): 193–198. https://doi.org/10.17265/2161-6256/2017.03.007
48. Islam A., Munro S., Hassan M. M., Epstein J. H., Klaassen M. The role of vaccination and environmental factors on outbreaks of high pathogenicity avian influenza H5N1 in Bangladesh. One Health. 2023; 17:100655. https://doi.org/10.1016/j.onehlt.2023.100655
49. Craig J. Why aren’t we vaccinating birds against bird flu? Vox. May 14, 2024. https://www.vox.com/future-perfect/24155545/bird-flu-vaccinesh5n1-avian-flu-cows
50. Pinto R. M., Bakshi S., Lytras S., Zakaria M. K., Swingler S., Worrell J. C., et al. BTN3A3 evasion promotes the zoonotic potential of influenza A viruses. Nature. 2023; 619 (7969): 338–347. https://doi.org/10.1038/s41586-023-06261-8
About the Authors
M. V. ZhiltsovaRussian Federation
Milena V. Zhiltsova, Cand. Sci. (Veterinary Medicine), Leading Researcher, Information and Analysis Centre
Yur’evets, Vladimir 600901
T. P. Akimova
Russian Federation
Tatiana P. Akimova, Leading Veterinarian, Information and Analysis Centre
Yur’evets, Vladimir 600901
M. N. Mitrofanova
Russian Federation
Mariya N. Mitrofanova, Cand. Sci. (Veterinary Medicine), Researcher, Information and Analysis Centre
Yur’evets, Vladimir 600901
V. P. Semakina
Russian Federation
Valentina P. Semakina, Head of Sector, Information and Analysis Centre
Yur’evets, Vladimir 600901
E. S. Vystavkina
Russian Federation
Evgeniya S. Vystavkina, Leading Specialist, Information and Analysis Centre
Yur’evets, Vladimir 600901
Review
For citations:
Zhiltsova M.V., Akimova T.P., Mitrofanova M.N., Semakina V.P., Vystavkina E.S. Extension of scope of susceptible mammalian species as avian influenza global situation developed in 2023–2024. Veterinary Science Today. 2025;14(1):6-13. https://doi.org/10.29326/2304-196X-2025-14-1-6-13



































