Scroll to:
BVDV comprehensive studies and species identification in high-yielding livestock populations in the Sverdlovsk Oblast
https://doi.org/10.29326/2304-196X-2024-13-4-330-337
Abstract
The paper presents results of comprehensive studies of the bovine viral diarrhea virus circulating in cattle populations in the Sverdlovsk Oblast. In 2018–2024, 113 biological samples were tested using polymerase chain reaction, the viral RNA specific regions were detected in 15.9% of cases.The BVDV RNA was isolated from biological samples collected from aborted cows (61.1%) and calves under one month of age (38.9%). Based on typing results, the virus isolates detected in four samples (nasopharyngeal swabs of calves, suspension prepared from aborted fetus organs and placenta) were classified as BVDV-1 virulent genotypes. The BVDV RNA, Mycoplasma bovis and Chlamydophila pecorum DNAs were detected simultaneously in 44% of vaginal swab samples from aborted cows and, in single cases, in the placenta and parenchymatous organs of dead calves; BVDV RNA and Bovine herpesvirus type 1 DNA were detected in 16% of pathological samples from dead calves. In some cases, the BVDV RNA, Chlamydophila pecorum and Mycoplasma bovigenitalium DNA were detected in nasopharyngeal swabs of calves. The “Comprehensive Programme for Biosecurity and Bovine Viral Diarrhea Situation Improvement in Agricultural Organizations”implemented in the Sverdlovsk Oblast in 2018 resulted in decreased number of agricultural establishments affected by bovine viral diarrhea. Acute and persistent infection forms among young animals were recorded 4 and 3.5 times less frequently, respectively, but at the same time, a 2.5-fold increase in the diagnosed latent form of the disease was observed in adult livestock, which is associated with an increase in the number of laboratory tests performed.
Keywords
For citations:
Bezborodova N.A., Kozhukhovskaya V.V., Pechura E.V., Martynov N.A., Tomskikh O.G., Vasilyeva A.N. BVDV comprehensive studies and species identification in high-yielding livestock populations in the Sverdlovsk Oblast. Veterinary Science Today. 2024;13(4):330-337. https://doi.org/10.29326/2304-196X-2024-13-4-330-337
INTRODUCTION
Bovine viral diarrhea (BVD) is classified as Сlass B infectious disease according to the World Organisation for Animal Health (WOAH). Official programmes aimed at BVD epizootological control and cattle population health improvement are implemented in the USA, UK and most European countries [1].
Establishing high-yielding dairy cattle herds is considered one of top priorities in the livestock industry of our country within achieving key objectives of the Strategy for Scientific and Technological Development of the Russian Federation (point 21g) [2]. However, viral infectious diseases negatively impact livestock industry development in the Russian Federation, same as in foreign countries [3-5].
Bovine viral diarrhea-induced economic losses for commercial and breeding farms include abortions, birth of weak and non-viable progeny, emergency slaughter of calves, reduced milk yields and shorter life expectancy of livestock [6]. The mortality level in BDV-infected farms is high (up to 10%). Asymptomatic occurrence of BVD in herds aggravates the situation at agricultural establishments, leading to total herd infection, decreased productivity and reproduction, and increased costs on treatment measures [6][7].
According to both Russian and foreign researchers, it is possible to reduce economic losses by regular activities on livestock health improvement and viral infection prevention [8-10]. The process of improving the BVD situation in livestock establishments takes a very long time and is not always successful due to the pathogenetic properties of the agent [8].
Bovine viral diarrhea virus (BVDV) is classified into the following species: BVDV-1, BVDV-2, HoBi-like (atypical) ruminant pestivirus, which are subdivided into subgenotypes based on phylogenetic analysis [11][12]. BVDV can be divided into cytopathic (CP) and non-cytopathic (NCP) biotypes [13][14]. The cytopathic biotype of BVDV can induce different cytopathological effects (CPE) in the host cell systems: cytoplasmic vacuolization and cell death, that are not observed for NCP biotypes of the virus [15]. Since the fetal immune system is immature, NCP BVDVs (BVDV-1, BVDV-2) inhibit the induction of type I interferon. Thus, NCP BVDV-1 or BVDV-2 may cause abortion or stillbirth in cows at early pregnancy stage [13-16].
Pestivirus type 1 (BVDV-1) is considered predominant among widespread cattle pathogens worldwide, while type 2 (BVDV-2) is more frequently reported in the USA and Canada, less frequently in Japan, India, South America and occasionally in European countries [17]. The emergence of new strains as well as BVDV strains with higher virulence, such as those causing haemorrhagic disease in animals (North America, genotype 2), emphasizes the need for diagnostic tests associated with virus genotyping [18]. The dose and virulence of the pathogen strains, the animal age and immunocompetence are the factors determining the pathogenicity level [19].
Faeces and excreta of animals containing a large number of viral particles are one of the important sources of infection [13]. The intestinal form of BVD is manifested by high fever, anorexia, diarrhea, severe dehydration, and blood in the faeces. The disease is usually sporadic, the incubation period lasts 1–2 weeks, and the mortality rate is extremely high [14]. The typical clinical signs in cows with mucosal disease are haemorrhagic, necrotic and ulcerative lesions. In addition, BVD can be manifested by loss of intestinal crypts, erosions, ulcers and large-scale mucosal necrosis, partially or throughout the gastrointestinal tract [16]. Animals that are immunotolerant to BVDV, once infected, become life-time carriers of the virus constantly shedding it, and a source of infection in the animal population, complicating the eradication of the disease [20][21].
In addition, BVDV-1 is detected in semen, posing a great threat of vertical transmission and indicating the need for continuous testing of servicing bulls for virus carriage [22-24].
Since the BVD clinical manifestations are similar to those of other diseases such as mycoplasmosis, chlamydia, paratuberculosis, parainfluenza-3, infectious bovine rhinotracheitis, laboratory tests play a crucial role for diagnosis [1].
Recently, a large number of respiratory and intestinal disease cases detected in cattle reared in the Sverdlovsk Oblast, have occurred in the form of mixed infections, when the pathogenic agents are at the same time viruses, bacteria and fungi [1]. Due to that, the disease is characterized by a pronounced severe course and a clinical picture not typical of monoinfections, and most often bacterial infection predominates, which complicates the diagnostic process. Herpesvirus type 1 (BHV-1), BVDV, as well as chlamydia (Chlamydophila abortus, Chlamydophila pecorum), pathogenic mycoplasma species (Mycoplasma bovis, Mycoplasma bovigenitalium, Mycoplasma spp.), various bacteria and their associations are recognized as prevailing pathogens in the development of the above-mentioned pathological forms in cattle [5][25][26].
The aim of the work was to conduct a comprehensive study of circulating bovine viral diarrhea virus and associated pathogens, and perform BVDV species identification in cattle populations in the Sverdlovsk Oblast.
MATERIALS AND METHODS
The study was carried out in the Department of Monitoring and Forecasting of Infectious Animal Diseases and in the Laboratory for Microbiological and Molecular Genetic Test Methods of the Ural Veterinary Research Institute being the structural subdivision of the Ural Federal Agrarian Research Centre, Ural Branch of the Russian Academy of Sciences within the framework of Federal Assignment No. 0532-2021-0007 “Study of the structure of the antigen pattern of livestock emergent infectious pathogens, and biological features of the mechanisms of their interaction with the macroorganism”.
Comprehensive laboratory tests were conducted in 21 livestock breeding establishments in the Sverdlovsk Oblast, where BVD health improvement programmes were implemented in 2018–2024.
The following biological material samples collected from Holstein cattle were used for testing: blood and blood sera; cervical and vaginal swabs of cows; nasopharyngeal swabs of calves; semen of servicing bulls; placenta samples; pieces of internal organs from dead calves (liver, kidneys, lungs) and aborted fetuses (n = 113).
Real-time polymerase chain reaction (PCR) was performed according to the manufacturer’s instructions for the use of test-kits. Test-kits for detection of BVDV RNA (Izogen Laboratory LLC, Russia), Mycoplasma spp., Mycoplasma bovis and Mycoplasma bovigenitalium DNAs (Russia), BHV1 DNA (GenPak PCR Test BHV1), Chlamydophila abortus and Chlamydophila pecorum DNAs (VectorBest LLC, Russia) were used. Amplification was performed using QuantStudio 5 unit (Thermo Fisher Scientific Inc., USA).
Additionally, 16 biological samples tested positive for BVDV RNA were placed for ultra-low temperature storage (–70 °C) for further virus typing with PCR. Sample types: pooled samples of nasopharyngeal swabs of 20-day-old calves, blood sera, aborted fetuses, placenta.
For BVDV genotyping, the following was performed: RNA isolation from samples using the AmpliPrime® RIBO-Prep VET kit (NextBio LLC, Russia), reverse transcription PCR (RT-PCR), real-time amplification and electrophoresis. Synthetic oligonucleotides previously developed by C. Letellier and P. Kerkhofs were used in this study [18] (Table 1).
These primer pairs are specific for highly conserved regions of the 5’ UTR. The sequences of probes labelled with FAM and ROX dyes with a three nucleotide-difference allowed differentiation between BVDV genotypes I and II.
The cDNA was generated using a commercial reagent kit for reverse transcription with MMLV-RH (Diaem LLC, Russia) according to the provided amplification programme in compliance with the instructions for use.
After RT-PCR and cDNA generation, real-time PCR was performed using BioMaster HS-Taq PCR (2×) master mix reagents (LLC Diaem, Russia); 100 mM Tris-HCl, pH 8.5 (at 25 °C), 100 mM KCl, 0.4 mM each deoxynucleoside triphosphate, 4 mM MgCl2, 0.06 active units/µL Taq DNA polymerase, 0.2% Tween 20, HS-Taq DNA polymerase stabilisers. For optimisation, different concentrations of the primers were selected. Amplification was performed using CFX 96 Touch system (Bio-Rad Laboratories, Inc., USA), parameters are shown in Table 2.
The RNAs isolated from the veterinary medicinal product Bovi-shield Gold FP5 L5 (Zoetis Inc., USA) that contained attenuated viruses of infectious bovine rhinotracheitis (Bovine herpesvirus, type 1), diarrhea (BVDV, types 1 and 2), parainfluenza-3 (PIV-3), respiratory syncytial infection (BRSV) were used as controls.
The PCR product was separated by gel electrophoresis using agarose gel and Mini-Sub Cell GT with visualisation in the ChemiDoc XRS+ camera and interpretation of results using Gel Doc XR+ (Bio-Rad Laboratories, Inc., USA). A 100 bp size standard (SibEnzyme, Russia) was used.
The obtained data were processed using Microsoft Excel within the Microsoft OfficePro 19 software package.
Table 1
Primer nucleotide sequences
|
Target |
Sequence 5’-3’ |
Product length |
|
BVDV_F |
CTCGAGATGCCATGTGGAC |
172 bp |
|
BVDV_PESTER5 |
CTCCATGTGCCATGTACAGCA |
|
|
BVDV_I |
FAM-CAGCCTGATAGGGTGCTGCAGAGGC-BHQ1 |
|
|
BVDV_II |
ROX-CACAGCCTGATAGGGTGTAGCAGAGACCTG-BHQ2 |
Table 2
Amplification programme
|
Temperature |
Time |
Number of cycles |
|
95 °С |
5 min |
1 |
|
94 °С |
20 sec |
40 (FAM/ROX) |
|
56 °С |
20 sec |
|
|
72 °С |
20 sec |
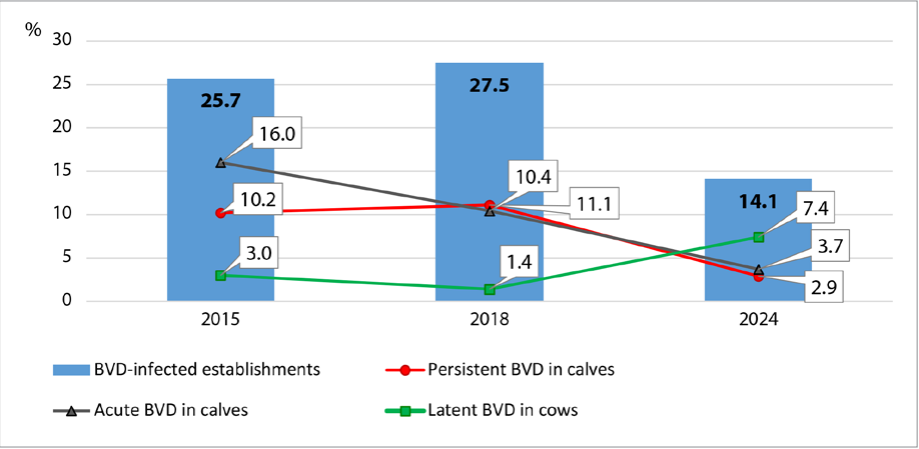
Fig. 1. BVD transmission dynamics in establishments in the Sverdlovsk Oblast (2015–2024)
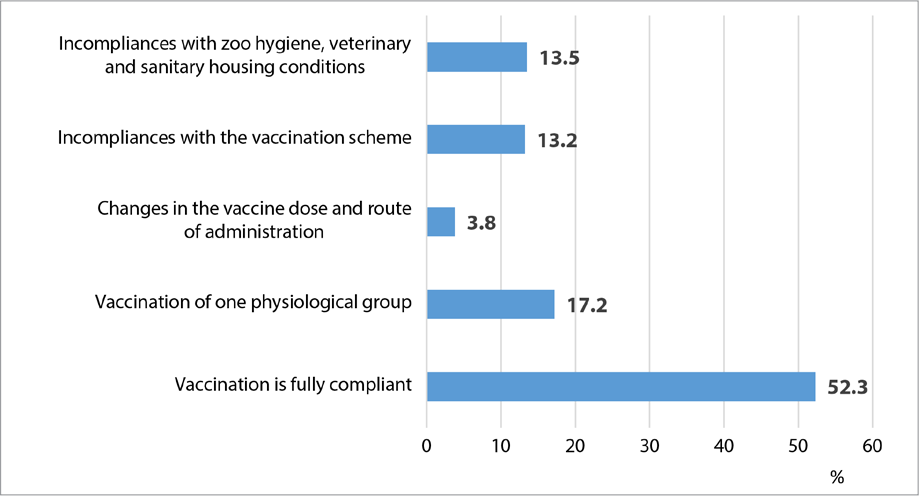
Fig. 2. Assessment of Animal Health Improvement Programme implementation
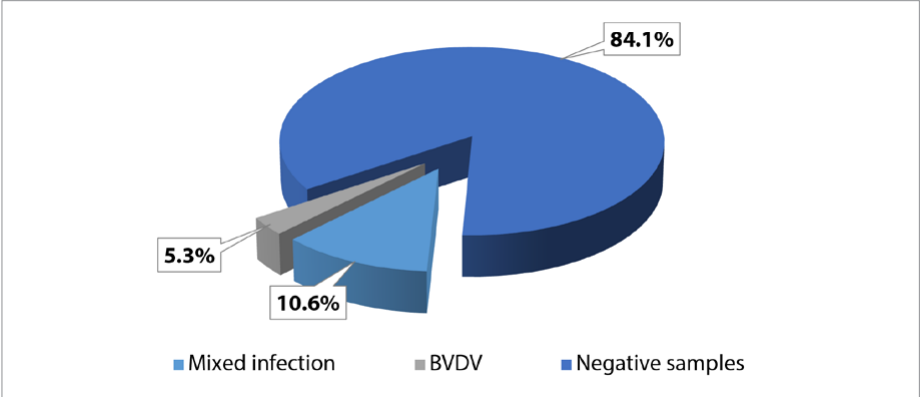
Fig. 3. PCR results for biological samples tested for presence of BVDV RNA and agents of mixed co-infections (2018–2024), n = 113
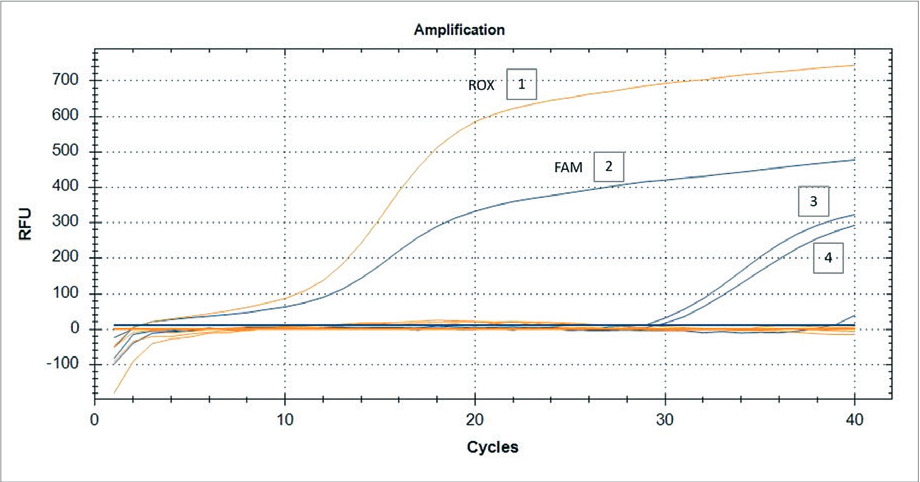
Fig. 4. Typing of BVDV detected in pooled samples of nasopharyngeal swabs from calves using RT-PCR, n = 6. Fluorescence curves: 1, 2 – control plus (FAM channel – BVDV-1; ROX channel – BVDV-2); 3, 4 – positive samples (FAM channel – BVDV-1)
RESULTS AND DISCUSSION
The implementation of the “Comprehensive Programme for Biosecurity and Bovine Viral Diarrhea Situation Improvement in Agricultural Organizations” began in the Sverdlovsk Oblast in 2018. The results of studies on the BVD transmission dynamics in the establishments of the Sverdlovsk Oblast previously published by O. V. Sokolova [15] have been supplemented with new data and are presented in Figure 1.
During the health improvement programme implementation a trend for decrease in the number of BVD-infected cattle farms was observed. In young animals, acute and persistent forms of infection were registered 4 and 3.5 times less frequently, respectively. It should be noted that there was a 2.5-fold increase in the diagnosed latent form of the disease in adult stock, which is associated with an increase in the level of laboratory diagnostic testing at agricultural establishments. At the same time, there were incompliances with the vaccination requirements detected. Thus, the analysis of the step-by-step implementation of the health improvement programme in farms showed that 47.7% of deficiencies occurred due to the human factor (Fig. 2).
As for the establishments where incompliances with vaccination schedules and inconsistent vaccination of physiological groups were detected, the latent BVD in adult cattle was registered in 67% cases of all tested samples.
Testing of 113 biological samples from cattle with diseases of the reproductive system, respiratory and gastrointestinal tract of unclear etiology received from 21 agricultural organizations in 2018–2024 showed that specific BVDV RNA sites were detected in 15.9% of cases: mixed infection – 10.6%, BVDV – 5.3% (Fig. 3).
Bovine viral diarrhea virus RNA was detected in 61.1% of biological samples from cows aborted at different stages of gestation, as well as in 38.9% of samples from calves.
As for samples of adult cows aborted at different stages of gestation, BVDV genetic material was more frequently detected in the homogenate of organs of aborted fetuses (38.8%), in vaginal swabs and in the placenta (11.2%). Also, the BVDV genome was detected in 27.7% cases in nasal swabs of calves with signs of acute respiratory and gastrointestinal diseases of unclear etiology, and in 11.1% of cases – in parenchymatous organs (suspensions of liver, heart, spleen, kidney and small intestine) of dead calves.
Additional BVDV typing tests using a primer system revealed the presence of genotype I virus in 4 out of 16 biological samples (nasopharyngeal swabs from 20-day-old calves, suspension of organs of aborted fetuses and placenta). Genotype II virus was not detected in the tested samples. It can be assumed that the BVDV-1 isolate (nasopharyngeal swabs of calves, aborted fetuses and placentas) is highly virulent, since the mortality of young animals was up to 20% and the number/frequency of abortions in dairy cattle was up to 5%.
The results of BVDV typing with RT-PCR are presented in Figure 4.
In 10.6% of samples, other infectious agents besides BVDV were detected. BVDV RNA, Mycoplasma bovis and Chlamydophila pecorum DNA were simultaneously detected in 44.0% of vaginal swabs from aborted cows and in single cases in placenta and parenchymatous organs from dead calves. Several pathogens were also detected in 16.0% of the samples from dead calves: BVDV + Bovine herpesvirus type 1. In single cases, BVDV RNA, Chlamydophila pecorum and Mycoplasma bovigenitalium DNA were detected in nasopharyngeal swabs of calves.
Thus, all measures to be implemented, including monitoring tests, diagnostic testing in “sentinel” animal groups, establishment of “closed-type herds”, animal vaccination and biosecurity, detection of latent infection carriers and timely treatment measures, are considered effective, and further monitoring of their implementation quality and removal of deficiencies will ensure BVD health improvement in the conditions of commercial
establishments.
CONCLUSION
Polymerase chain reaction tests of 113 biological samples collected from cows and calves in 2018–2024 demonstrated presence of BVDV RNA in 15.9% of cases. The BVDV genome was detected in samples from cows aborted at different gestation stages in 61.1% of cases and from calves in 38.9% of cases. The virus typing showed its belonging to type 1 (in 4 samples: nasopharyngeal swabs from calves, suspension from organs of aborted fetuses and placenta). In addition to BVDV, other infectious agents were detected in 10.6% of samples, indicating the presence of mixed infection. Combinations of BVDV with such pathogens as Mycoplasma bovis, Mycoplasma bovigenitalium, Chlamydophila pecorum, Bovine herpesvirus (type 1) were detected.
Monitoring diagnostic tests indicate positive dynamics in reduction of BVD cases in the establishments of the Sverdlovsk Oblast, as well as demonstrate successful implementation of the “Comprehensive Programme for Biosecurity and Bovine Viral Diarrhea Situation Improvement in Agricultural Organizations”. Currently, there is a 2-fold decrease in the number of BVD-infected farms, while the share of detected latent infection cases in adult stock increased by 2.5 times, which is due to the extension of the diagnostic test panel, regular monitoring of herds, as well as incompliances with vaccination procedure during this programme implementation.
Further monitoring of the quality of the programme implementation, including clinical and laboratory tests aimed at detecting BVDV circulation and eliminating incompliances with vaccination regulations, will ensure health improvement of herds at commercial establishments.
Work is to be continued on BVDV typing, which will include quantification of the virus genomes and sequencing of BVDV circulating in animal herds in the Sverdlovsk Oblast for subsequent genetic analysis (subtyping, tests for virulence of isolates).
References
1. Bezborodova N. A., Kozhukhovskaya V. V., Poryvaeva A. P., Shilova E. N., Pechura E. V. The importance of PCR in the infectious agents detection in cattle. Bulletin of KSAU. 2022; (12): 127–133. https://elibrary.ru/krzily (in Russ.)
2. About the strategy of scientific and technological development of the Russian Federation: Decree of the President of the Russian Federation No. 145 of 28 February 2024. https://www.garant.ru/products/ipo/prime/doc/408518353/?ysclid=m1z1siqrm7980575754 (in Russ.)
3. Chi S., Chen S., Jia W., He Y., Ren L., Wang X. Non-structural proteins of bovine viral diarrhea virus. Virus Genes. 2022; 58 (6): 491–500. https://doi.org/10.1007/s11262-022-01914-8
4. Koteneva S. V., Nefedchenko A. V., Glotova T. I., Sudorgina T. E., Semenova O. V., Glotov A. G. Detection of BVDV-1f in a respiratory disease outbreak in calves. Veterinariya. 2023; (8): 15–21. https://elibrary.ru/ohayby (in Russ.)
5. Semenova O. V., Koteneva S. V., Nefedchenko A. V., Sudorgina T. E., Glotova T. I., Glotov A. G. An outbreak of mucosal disease in cattle caused by Pestivirus H. Siberian Herald of Agricultural Science. 2023; 53 (4): 71–80. https://doi.org/10.26898/0370-8799-2023-4-8
6. Kozhukhovskaya V. V. Analysis of the spread of viral diarrhea in cattle in the Ural Region. Nauchnye dostizheniya genetiki i biotekhnologii v veterinarnoi meditsine i zhivotnovodstve: sbornik materialov nauchnoprakticheskoi konferentsii s mezhdunarodnym uchastiem (Ekaterinburg, 27–28 maya 2021 g.) = Scientific achievements of genetics and biotechnology in veterinary medicine and animal husbandry: collection of proceedings from a scientifi and practical conference with international participation (Ekaterinburg, May 27–28, 2021). Ekaterinburg: Ural Federal Agrarian Scientific Research Center, Ural Branch of the Russian Academy of Sciences; 2021; 53–58. https://elibrary.ru/bxbqyb (in Russ.)
7. Poryvaeva A. P., Pechura E. V., Petrova O. G., Bezborodova N. A., Lysova Ya. Yu., Belousova D. A. The effectiveness of scientifically based monitoring programs and therapeutic and preventive measures for controlled infectious diseases of animals. International Bulletin of Veterinary Medicine. 2023; (4): 96–110. https://doi.org/10.52419/issn2072-2419.2023.4.96 (in Russ.)
8. Glotov A. G., Glotova T. I., Nefedchenko A. V., Grebennikova T. V., Aliper T. I. The use of PCR for the diagnosis of viral diarrhea – disease of shell mucous in cattle. Veterinariya. 2007; (12): 27–29. https://elibrary.ru/iirtuh (in Russ.)
9. Su A., Fu Y., Meens J., Yang W., Meng F., Herrler G., Becher P. Infection of polarized bovine respiratory epithelial cells by bovine viral diarrhea virus (BVDV). Virulence. 2021; 12 (1): 177–187. https://doi.org/10.1080/21505594.2020.1854539
10. Oguejiofor C. F., Thomas C., Cheng Z., Wathes D. C. Mechanisms linking bovine viral diarrhea virus (BVDV) infection with infertility in cattle. Animal Health Research Reviews. 2019; 20 (1): 72–85. https://doi.org/10.1017/S1466252319000057
11. Bielefeldt-Ohmann H. Special issue: Bovine viral diarrhea virus and related pestiviruses. Viruses. 2020; 12 (10):1181. https://doi.org/10.3390/v12101181
12. Goto Y., Yaegashi G., Fukunari K., Suzuki T. An importance of longterm clinical analysis to accurately diagnose calves persistently and acutely infected by bovine viral diarrhea virus 2. Viruses. 2021; 13 (12):2431. https://doi.org/10.3390/v13122431
13. Klimowicz-Bodys M. D., Polak M. P., Płoneczka-Janeczko K., Bagnicka E., Zbroja D., Rypuła K. Lack of fetal protection against bovine viral diarrhea virus in a vaccinated heifer. Viruses. 2022; 14 (2):311. https://doi.org/10.3390/v14020311
14. Li Y., Liu T., Chen G., Wang L., Guo A., Li Z., et al. Th17 cell differentiation induced by cytopathogenic biotype BVDV-2 in bovine PBLCs. BMC Genomics. 2021; 22:884. https://doi.org/10.1186/s12864-021-08194-w
15. Sokolova O. V. Morphofunctional changes in the mother-placentafetus system in cows with viral, bacterial and protozoal infections: Author’s Thesis for Degree of Dr. Sci. (Veterinary Medicine). Ekaterinburg; 2020. 287 p. (in Russ.)
16. Bianchi M. V., Silveira S., Mósena A. C. S., de Souza S. O., Konradt G., Canal C. W., et al. Pathological and virological features of skin lesions caused by BVDV in cattle. Brazilian Journal of Microbiology. 2019; 50 (1): 271–277. https://doi.org/10.1007/s42770-018-0019-0
17. Wang W., Shi X., Tong Q., Wu Y., Xia M. Q., Ji Y., et al. A bovine viral diarrhea virus type 1a strain in China: isolation, identifi tion, and experimental infection in calves. Virology Journal. 2014; 11:8. https://doi.org/10.1186/1743-422X-11-8
18. Letellier C., Kerkhofs P. Real-time PCR for simultaneous detection and genotyping of bovine viral diarrhea virus. Journal of Virological Methods. 2003; 114 (1): 21–27. https://doi.org/10.1016/j.jviromet.2003.08.004
19. Nefedchenko A. V., Glotov A. G., Glotova T. I., Kungurtseva O. V. Detection of cattle persistently infected by bovine viral diarrhea virus with PCR. Veterinariya. 2011; (12): 21–25. https://elibrary.ru/oouolv (in Russ.)
20. Quintana M. E., Barone L. J., Trotta M. V., Turco C., Mansilla F. C., Capozzo A. V., Cardoso N. P. In-vivo activity of IFN-λ and IFN-α against bovine-viral-diarrhea virus in a mouse model. Frontiers in Veterinary Science. 2020; 7:45. https://doi.org/10.3389/fvets.2020.00045
21. Read A. J., Gestier S., Parrish K., Finlaison D. S., Gu X., O’Connor T. W., Kirkland P. D. Prolonged detection of bovine viral diarrhoea virus infection in the semen of bulls. Viruses. 2020; 12 (6):674. https://doi.org/10.3390/v12060674
22. Liu C., Liu Y., Liang L., Cui S., Zhang Y. RNA-Seq based transcriptome analysis during bovine viral diarrhoea virus (BVDV) infection. BMCGenomics. 2019; 20 (1):774. https://doi.org/10.1186/s12864-019-6120-4
23. Newcomer B. W. 75 years of bovine viral diarrhea virus: Current status and future applications of the use of directed antivirals. Antiviral Research. 2021; 196:105205. https://doi.org/10.1016/j.antiviral.2021.105205
24. Yao R., Xu Y., Wang L., Wang D., Ren L., Ren C., et al. CRISPR-Cas13abased detection for bovine viral diarrhea virus. Frontiers in Veterinary Science. 2021; 8:603919. https://doi.org/10.3389/fvets.2021.603919
25. Jokar M., Rahmanian V., Farhoodi M., Abdous A., Shams F., Karami N. Seroprevalence of bovine viral diarrhea virus (BVDV) infection in cattle population in Iran: a systematic review and meta-analysis. Tropical Animal Health and Production. 2021; 53 (5):449. https://doi.org/10.1007/s11250021-02918-6
26. Koteneva S. V., Glotova T. I., Nefedchenko A. V., Glotov A. G. An outbreak coronavirus infection with respiratory syndrome in calves at the big dairy farm. Veterinariya. 2023; (1): 16–22. https://doi.org/10.30896/00424846.2023.26.1.16-23 (in Russ.)
About the Authors
N. A. BezborodovaRussian Federation
Natalia A. Bezborodova, Cand. Sci. (Veterinary Medicine), Senior Researcher, Department of Animal Genomics and Selection
112а Belinsky str., Ekaterinburg 620142, Russia
V. V. Kozhukhovskaya
Russian Federation
Veronika V. Kozhukhovskaya, Junior Researcher, Department of Veterinary Laboratory Diagnostics with a Testing Laboratory
112а Belinsky str., Ekaterinburg 620142, Russia
E. V. Pechura
Russian Federation
Elena V. Pechura, Dr. Sci. (Veterinary Medicine), Leading Researcher, Department of Monitoring and Forecasting of Infectious Animal Diseases
112а Belinsky str., Ekaterinburg 620142, Russia
N. A. Martynov
Russian Federation
Nikolay A. Martynov, Laboratory Assistant, Department of Animal Genomics and Selection
112а Belinsky str., Ekaterinburg 620142, Russia
O G. Tomskikh
Russian Federation
Oksana G. Tomskikh, Cand. Sci. (Veterinary Medicine), Senior Researcher, Department of Monitoring and Forecasting of Infectious Animal Diseases
112а Belinsky str., Ekaterinburg 620142, Russia
A. N. Vasilyeva
Russian Federation
Anna N. Vasilyeva, Junior Researcher, Department of Veterinary Laboratory Diagnostics with a Testing Laboratory
112а Belinsky str., Ekaterinburg 620142, Russia
Review
For citations:
Bezborodova N.A., Kozhukhovskaya V.V., Pechura E.V., Martynov N.A., Tomskikh O.G., Vasilyeva A.N. BVDV comprehensive studies and species identification in high-yielding livestock populations in the Sverdlovsk Oblast. Veterinary Science Today. 2024;13(4):330-337. https://doi.org/10.29326/2304-196X-2024-13-4-330-337
JATS XML



































