Scroll to:
Astrovirus infection in animals (literature review)
https://doi.org/10.29326/2304-196X-2024-13-4-322-329
Abstract
Viral agents are a major cause of mass gastroenteritides in newborn calves in the countries around the world. Early postnatal diarrhea as the main reason of morbidity and mortality in young animals leads to serious problems in the commercial livestock farming and causes a considerable economic damage. The most common viral gastroenteritis agents in calves are rotaviruses, coronaviruses and pestiviruses, and, along with these, astroviruses are increasingly being detected. The members of the family Astroviridae can cause various pathologies in animals: enteritis, hepatitis and nephritis in birds, gastroenteritis, neurological syndromes and encephalitis in mammals. The role of these viruses in the etiology of respiratory pathology in animals has been demonstrated. The following animals are the natural hosts of astrovirus: cattle, small ruminants, camels, deer, yaks, roe deer, buffaloes, alpacas, pigs, wild boars. The virus has been detected in bats, rodents and marine mammals, as well as in mollusks. Presently, the list of animals susceptible to astrovirus infection has expanded to over 80 species from 22 families, including domestic, synanthropic and wild animals, birds and mammals living in the terrestrial and aquatic environments. In recent times, there has been a lot of evidence of occurrence of recombinant astrovirus isolates, which contributes to the emergence of new genetic variants of the pathogen. A wide variety of infected animal species, the genetic diversity of the virus and the recombination events are indicative either of the cross-species transmission and subsequent adaptation of the virus to new hosts, or of the coinfection of the same host with different virus genotypes, which may lead to the emergence of novel astroviruses that are capable of infecting animals or possess a zoonotic potential. Astrovirus infection has no specific clinical features that allow for its differentiation from other intestinal infections. The presented data highlight the necessity for taking into account astrovirus infection when testing pathological material samples from diarrhea-affected newborn calves, lambs, goat kids and piglets on the commercial farms of the country.
Keywords
For citations:
Mischenko V.A., Mischenko A.V., Nikeshina Т.B., Petrova О.N. Astrovirus infection in animals (literature review). Veterinary Science Today. 2024;13(4):322-329. https://doi.org/10.29326/2304-196X-2024-13-4-322-329
INTRODUCTION
Gastrointestinal diseases of newborn calves and young cattle are widespread all over the world and second only to respiratory pathology in the extent of the economic damage caused [1-5]. Newborn calf diarrhea is the main cause of morbidity, mortality and economic losses in livestock farming [3][6-12]. Vaccines have been developed to prevent rotavirus, coronavirus infections and bovine viral diarrhea/mucosal disease (BVD-MD) [1-3][13][14]. However, diarrhea is sometimes reported in newborn calves born to vaccinated cows. Diagnosis in mass viral diarrhea cases is based on the detection of rotavirus, coronavirus and pestivirus (the causative agent of viral diarrhea) or postinfection antibodies. There is no difference in the clinical signs demonstrated by diseased newborn calves with diarrheas caused by bovine viral diarrhea/mucosal disease agents, rotaviruses, coronaviruses, kobuviruses, toroviruses, parvoviruses, enteroviruses, neboviruses, noroviruses, bopiviruses [3][8][11][14][15-18]. Also, no differences in the postmortem lesions were detected by the necropsy of calves that had died of rotavirus, coronavirus, parvovirus and enterovirus infections.
The negative results of laboratory tests aimed at the detection of the said pathogens prompted additional tests of pathological material samples using other diagnostic methods. Reports on the detection of astroviruses in the faecal samples from diarrhea-affected calves were published in 1977–1978 [19]. The astrovirus isolated from the faecal samples from calves with diarrhea in England was found to be antigenically related to the pathogen recovered from a diseased animal from Florida (USA). Then the targeted testing of faecal samples for astroviruses revealed the wide occurrence (46%) of the virus on livestock farms. On 88% of the tested farms, other pathogens (rotaviruses, coronaviruses, parvoviruses, noroviruses and enteroviruses) were detected along with astrovirus. In 8% of cases, only astroviruses were detected [5][19-21]. The members of the family Astroviridae can cause diseases in various vertebrates, with the isolates recovered from birds and mammals being the most well studied.
MAIN PART
Until recently, human pathology was thought to be associated with 8 serotypes of astroviruses (Human astrovirus, HAstV). However, in the last few years, the wide use of molecular biological test methods allowed for the detection of some more groups of the pathogen (MLB and VA) differing from conventional human astroviruses in patients with acute diarrhea manifestations. These groups of astroviruses are detected quite rarely, but can cause a group disease [22-24]. Astrovirus serotypes (genotypes) 1 and 2 are most common in children, and serotype 4 – in older persons. Astrovirus infection has no specific clinical features that allow for its differentiation from other intestinal infections. The contribution of this infection to the sporadic morbidity in different regions of the world varies widely (4–17%) [25][26].
Spherical non-enveloped virions with a size of about 28–30 nm were detected in the faecal samples from diarrhea-affected newborn calves born to cows immunized with vaccines against rotavirus, coronavirus infections and bovine viral diarrhea/mucosal disease. The surface of the virion resembled five- or six-pointed stars. Such virions were first detected using electron microscopy in 1975 during the tests of faecal samples from children with diarrhea; subsequently, similar viruses were found in faecal samples taken from diarrhea-affected animals of different species [27, 28]. The name of the detected virus comes from the Greek word “astron”, meaning “star”, which the virions resemble in electron micrographs (Fig. 1) [13][26][29].
In 1995, the International Committee on Taxonomy of Viruses assigned all astroviruses to the new family Astroviridae [30]. This family includes two genera: Mamastrovirus (from the Latin word “mamma”, meaning “mammary gland”) and Avastrovirus (from the Latin word “avis”, meaning “bird”) [25, 31, 32]. Viruses belonging to the genus Mamastrovirus cause pathology in humans and mammalian animals. The representatives of the genus Avastrovirus cause the disease in birds (Fig. 2). Methods such as electron microscopy, polymerase chain reaction and metagenomic analysis allowed for the detection of astroviruses in pathological material samples from domestic and wild animals, including cattle (Bovine astrovirus, BoAstV), camels (Dromedary camel astrovirus, DcAstV), sheep (Ovine astrovirus, OAstV), goats (Caprine astrovirus, CapAstV), pigs (Porcine astrovirus, PoAstV), dogs (Canine astrovirus, CaAstV), cats (Feline astrovirus, FeAstV), minks (Mink astrovirus,
MiAstV), mice (Murine astrovirus, MuAstV), rats (Rat astrovirus, RatAstV), dolphins (Bottlenose dolphin astrovirus, BdAstV), pinnipeds (California sea lion astroviruses, CslAstV; Steller sea lion astroviruses, SslAstV), chickens (Chicken astrovirus, CAstV), turkeys (Turkey astrovirus, TAstV), ducks (Duck astrovirus, DAstV), geese (Goose astrovirus, GAstV) and other animal species [25][28][33].
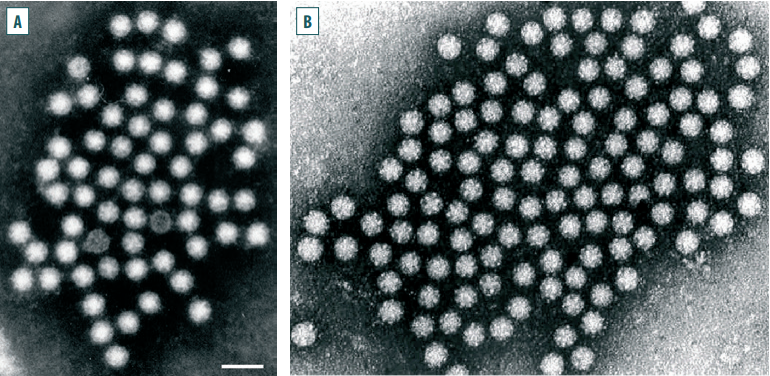
Fig. 1. An electron micrograph of an astrovirus: А – [26]; B – [29]
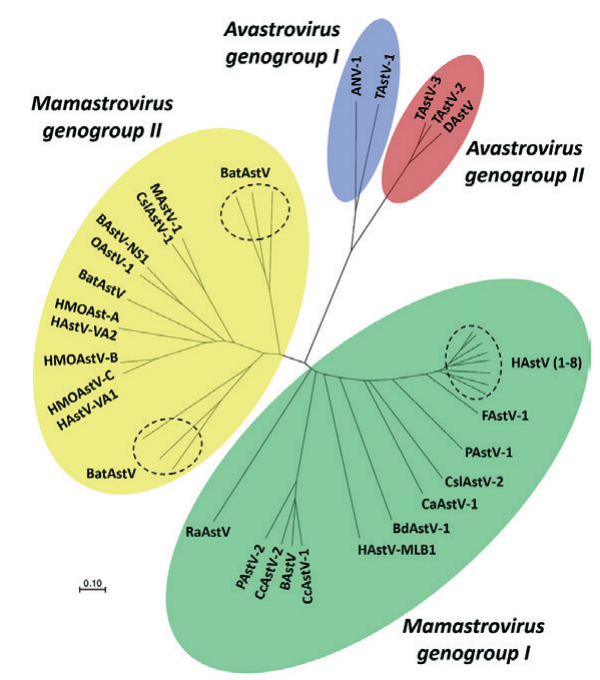
Fig. 2. Phylogenetic relationships within the family Astroviridae [28]
Astroviruses are represented by non-enveloped virions of icosahedral symmetry with a diameter of 28–30 nm. The nucleocapsid consists of three main capsid proteins. The virion capsid is covered with 30 dimeric spikes protruding above the virion surface at 3–8 nm. The molecular weight of the virion is 8 MDa. The astrovirus genome is represented by a single-stranded infectious RNA (6.4–7.9) × 103 nucleobases long, containing three open reading frames. The genomic sequence of bovine astroviruses was determined in 2011 [27][34-36]. The buoyant density of astrovirus in caesium chloride is 1.36–1.39 g/cm3. The sedimentation constant is 140S.
Astroviruses are inactivated at 50 °C for 60 minutes or at 60 °C for 5 minutes. The virus is stable at pH 3.0, as well as resistant to various detergents and fat solvents. In faecal samples from animals with astrovirus diarrhea, the pathogen was detected at a concentration of up to 1010 particles/mL. The natural hosts of astrovirus are cattle, small ruminants, camels, deer, yaks, roe deer, buffaloes, alpacas, pigs, wild boars. The pathogen has been detected in bats, rodents and marine mammals, as well as in mollusks. Presently, the list of animals susceptible to astrovirus infection has expanded to over 80 species from 22 families, including domestic, synanthropic and wild animals, birds and mammals living in the terrestrial and aquatic environments [25][37-43].
Astroviruses are transmitted via the faecal-oral route. The RNA of this pathogen is infectious, and after the virion enters the target cell, it serves as an mRNA for the translation of two non-structural proteins. Astroviruses replicate in the cytoplasm of sensitive cells, they destroy intestinal cells and release during lysis [2][13][27][44].
Astrovirus infection is geographically widespread throughout the world [6]. Genetic variability has been described for almost all sufficiently studied species of astroviruses infecting mammals and birds; however, antigenic variability has been demonstrated in human astrovirus, but is much less studied in animal viruses. In recent times, there has been a lot of evidence of occurrence of recombinant astrovirus isolates, which contributes to the higher genetic variation in this group of viruses. A wide variety of infected animal species, the evident genetic diversity of the viruses and the recombination events are indicative either of the cross-species transmission and subsequent adaptation of the virus to new hosts, or of the coinfection of the same host with different astroviruses. It is believed that the coinfection may lead to the emergence of novel astroviruses that are capable of infecting animals or possess a zoonotic potential [25][45][46].
It was found that an astrovirus can be cultivated in a primarily trypsinized human embryo cell culture when the serum-free maintenance medium is supplemented with 10 µg/mL of trypsin to activate the replication. In the absence of trypsin, the astrovirus entry into the target cells and their infection, as well as the release of the infectious pathogen do not occur. All this indicates that astrovirus replication is trypsin-dependent [1][47-49]. Astroviruses of cattle and small ruminants replicate in the primarily trypsinized cultures of calf embryo kidney cells, as well as in continuous cell lines (MDBK, BT, EBK, GBK). The astrovirus infection incubation period is 4.5 days [50]. The studies of the disease pathogenesis in newborn lambs showed that two-day-old animals developed diarrhea 48 hours after experimental infection [12][51]. In the body of newborn calves and lambs, astrovirus replicates in the enterocytes of the apical surface of the small intestine villi. After entering the intestines of newborn animals, the virus infects the enterocytes of the ileum and epithelial M cells of the epithelium of the dome of Peyer’s patches [34][44][52][53]. Despite astrovirus detection in faeces, in some cases calves had no clinical signs of the disease.
Bovine astroviruses were detected in 60% of faecal samples taken from newborn calves with diarrhea on Brazilian farms. According to the phylogenetic analysis data, the detected isolates demonstrated a 74.3–96.5% similarity based on the amino acid sequence [47].
Chinese scientists conducted polymerase chain reaction tests of 211 rectal swab samples from cattle and water buffaloes with the signs of diarrhea living in the same ecocluster. Astrovirus RNA was detected in 46.10% of the samples from cattle and in 36.84% of the samples from buffaloes. The phylogenetic analysis results indicate that the pathogens had a common ancestor [34]. In the pastures of Tibet, diarrhea of newborn cattle and yak calves is the most common disease causing significant economic damage. The tests of the faecal samples collected from young yaks revealed the presence of the following viruses: rotavirus, parvovirus, astrovirus, nebovirus, enterovirus, influenza A virus, hepatitis E virus, kobuvirus and bovine viral diarrhea/mucosal disease virus. The astrovirus isolated from the faecal samples from yaks was 46.4–66.2% identical to the virus isolated from the faeces of diseased cattle. The test results showed that yak astroviruses belong to the cluster of bovine astroviruses. However, yak astroviruses demonstrate a more close genetic relatedness to deer astroviruses. The researchers suggested that interspecies recombination had occurred in the astrovirus ORF2. All this indicates that the pathogen isolated from the faeces of newborn yak calves with diarrhea is a novel astrovirus [9]. In South Korea, the studies of diarrhea etiology in 115 newborn calves from different farms revealed the presence of astroviruses in 7.83% of samples [36]. Zhu J. et al. detected astrovirus both in the faecal samples taken from clinically healthy calves and in the samples from calves with diarrhea [54].
Astroviruses were detected in 3.15% of faecal samples from newborn calves with diarrhea in three provinces of Central Turkey. Based on the phylogenetic analysis data, the detected new strains of astrovirus were 75.8–100.0% identical [55]. In 2012–2013, faecal samples were collected on 36 farms in Scotland to investigate the etiology of mass diarrhea cases in newborn calves. Astroviruses were detected in 80.0% of the samples from diseased calves, and rotaviruses were detected in 77.1% of the samples. Astroviruses (64.4%) and rotaviruses (17.8%) were also detected in faecal samples from clinically healthy calves. Astroviruses were isolated from 15.0% of the faecal samples taken from adult cattle from the same farms. The detected astroviruses belonged to three genetic lineages [21].
Polymerase chain reaction tests of faecal samples from 25 calves with diarrhea from two farms in Egypt detected rotaviruses in 48% of the samples, noroviruses in 24% of the samples and astroviruses in 32% of the samples. Two pathogens were detected in 37% of the samples. The results of these studies indicate a high degree of similarity in the nucleotide sequences of the Egyptian and Brazilian isolates of bovine astroviruses [56]. Astroviruses were detected in faecal samples from diarrhea-affected sheep from different countries [12][51][53]. When studying the etiology of diarrhea in animals, Swiss researchers detected astroviruses in faecal samples from 10.7% of lambs, 14.3% of goat kids, 10.0% of alpacas and 16.7% of fawns. High genetic similarity between ovine and caprine astroviruses is indicative of the multiplicity of the pathogen transmission pathways [53].
Astroviruses detected in the samples collected from marine mammals (a sea lion, dolphins) were found to be related to the viruses detected in the samples taken from terrestrial animals. This diversity of marine mammal astroviruses and their similarity to terrestrial animal astroviruses suggest that the marine environment plays an important role in the ecology of the pathogen [40][41].
Japanese researchers conducted a metagenomic analysis of 146 faecal samples collected from calves with diarrhea in the period from 2009 to 2015 in three prefectures of the country. Astroviruses were detected in 15 samples. Based on the phylogenetic analysis data, 9 astrovirus isolates were found to be similar to the Chinese isolates and were classified as belonging to lineage 1. Three strains were classified as belonging to the group of American strains isolated from cattle with respiratory pathology (lineage 2). One isolate was classified as belonging to a separate group along with type 5 porcine astrovirus and ovine astrovirus. The results of these studies served as the basis for the assumption of the existence of the interspecies transmission of astroviruses [35]. The investigation of the causes of diarrhea in the European population (Danish population) revealed the presence of astroviruses belonging to types 1 and 2 of the pathogen in faecal samples [21]. Astroviruses were also detected in faecal samples from European roe deer, red and white-tailed deer [42][43][57][58]. The phylogenetic analysis results demonstrate a close genetic relatedness of the strains isolated from roe deer. These pathogens were also related to the astroviruses isolated from cattle, deer, water buffaloes, yaks, two-humped (Bactrian) camels, Sichuan takins, pigs and porcupines [57].
Reverse transcription polymerase chain reaction tests of 215 faecal samples taken from one-humped (dromedary) camels in the United Arab Emirates detected astrovirus (DcAstV) in 4 of them. Camel astroviruses were found to belong to a separate cluster of pathogens, which are 60–66% related to type 2 porcine astroviruses. These data served as the basis for the assumption that one-humped camels are a natural reservoir in which the astrovirus has steadily evolved. Camel astroviruses are a novel species of the genus Mamastrovirus of the family Astroviridae [33].
Several countries reported cattle cases with central nervous system disorders. Tests of the brain samples (Fig. 3) taken from the corpses of diseased animals detected an astrovirus [59-62]. The role of astroviruses in the etiology of respiratory pathology in animals was demonstrated [35][63][64]. A number of researchers classify astroviruses as pathogens with a zoonotic potential [25][28][38][52][65]. All this indicates the importance of the timely diagnosis of astrovirus infection. Currently, all modern immunochemical and molecular biological methods are used to diagnose this infection in animals [51][61][66].
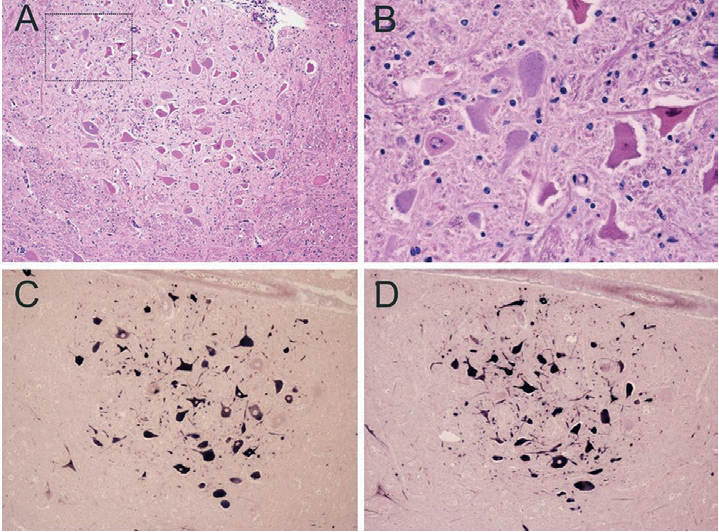
Fig. 3. Histopathological changes and detection of BoAstV RNA in the affected brain tissues of a cow with non-suppurative encephalitis: А – gliosis and neuronal necrosis; В – necrotic neurons (magnification of the marked area in panel A); С and D – dark blue labelling indicates the presence of the viral RNA [59]
CONCLUSION
Astrovirus infection is considered to be one of the most common causes of mass gastroenteritides in various animal species in many countries of the world. The role of astroviruses in the etiology of respiratory pathology in cattle has been determined. The disease cases in cattle characterized by central nervous system disorders have been reported. The results of molecular biological tests of astrovirus isolates recovered from pathological material samples indicate the genetic variability of the virus. There are regular reports of recombinations detected in astroviruses. A wide variety of infected animal species and the occurrence of recombinations are indicative of the cross-species transmission and subsequent adaptation of the astrovirus to new hosts or of the coinfection of the same host with different viruses. This can also lead to the emergence of novel astroviruses that infect animals and possess a zoonotic potential. The presented data highlight the necessity for taking into account astrovirus infection when testing pathological material samples from diarrhea-affected newborn calves, lambs, goat kids and piglets on the commercial farms of the country. Astrovirus infection should also be taken into consideration when examining pathological material samples collected from animals with respiratory pathology. Data on recombination events occurring between human astroviruses and animal astroviruses suggest the possibility of formation of new groups of viruses potentially capable of causing the disease in animals. Feed and water, as well as animal care tools contaminated with the pathogen can serve as astrovirus transmission factors. All this indicates the need to take into account astrovirus infection when conducting epizootiological investigations and identifying the etiology of mass cases of gastrointestinal pathology in newborn calves, piglets, lambs, goat kids, foals, dogs and cats.
References
1. Current infectious diseases of cattle: a manual. Ed. by T. I. Aliper. Moscow: Sel’skokhozyaistvennye tekhnologii; 2021. 832 p. (in Russ.)
2. Gaffarov Kh. Z., Ivanov A. V., Nepoklonov E. A., Ravilov A. Z. Monoand mixed infectious diarrhea of neonatal calves and piglets. Kazan: Fen; 2002. 592 p. (in Russ.)
3. Guidance on virology. Human and animal viruses and viral infections. Ed. by D. K. Lvov. Moscow: Meditsinskoe informatsionnoe agentstvo; 2013; 274–276. (in Russ.)
4. Chernykh O. Yu., Shevchenko A. A., Mishchenko V. A., Mishchenko A. V., Shevkoplyas V. N. Аstrovirus infection of cattle. Proceedings of the Kuban State Agrarian University. 2015; (57): 156–166. https://elibrary.ru/whwydv (in Russ.)
5. Yurov K. P., Gulyukin M. I., Mnikova L. A., Alexeyenkova S. V., Ishkova T. A. Viruses causing frequent and emergent gastrointestinal infections of cattle (review). Veterinaria i kormlenie. 2021; (2): 55–58. https://doi.org/10.30917/ATT-VK-1814-9588-2021-2-15 (in Russ.)
6. Caffarena R. D., Casaux M. L., Schild C. O., Fraga M., Castells M., Colina R., et al. Causes of neonatal calf diarrhea and mortality in pasture-based dairy herds in Uruguay: A farm-matched case-control study. Brazilian Journal of Microbiology. 2021; 52 (2): 977–988. https://doi.org/10.1007/s42770021-00440-3
7. Capozza P., Martella V., Lanave G., Catella C., Diakoudi G., Beikpour F., et al. An outbreak of neonatal enteritis in buffalo calves associated with astrovirus. Journal of Veterinary Science. 2021; 22 (6):e84. https://doi.org/10.4142/jvs.2021.22.e84
8. Castells M., Colina R. Viral enteritis in cattle: to well known viruses and beyond. Microbiology Research. 2021; 12 (3): 663–682. https://doi.org/10.3390/microbiolres12030048
9. Chen X., Zhang B., Yue H., Wang Y., Zhou F., Zhang Q., Tang C. A novel astrovirus species in the gut of yaks with diarrhoea in the Qinghai-Tibetan Plateau, 2013. Journal of General Virology. 2015; 96 (12): 3672–3680. https://doi.org/10.1099/jgv.0.000303
10. Cho Y. I., Yoon K. J. An overview of calf diarrhea – infectious etiology, diagnosis, and intervention. Journal of Veterinary Science. 2014; 15 (1): 1–17. https://doi.org/10.4142/jvs.2014.15.1.1
11. Gomez D. E., Weese J. S. Viral enteritis in calves. The Canadian Veterinary Journal. 2017; 58 (12): 1267–1274. https://pubmed.ncbi.nlm.nih.gov/29203935
12. Herring A. J., Gray E. W., Snodgrass D. R. Purification and characterization of ovine astrovirus. Journal of General Virology. 1981; 53 (Pt. 1): 47–55. https://doi.org/10.1099/0022-1317-53-1-47
13. Sergeev V. A., Nepoklonov E. A., Aliper T. I. Viruses and viral vaccines. Moscow: Biblionika; 2007; 467–469. (in Russ.)
14. Mischenko V. A., Getmanskyi O. I., Nikeshina T. B., Dumova V. V., Pavlov D. K., Zhbanova T. V., et al. Effectiveness of preventive vaccination against bovine neonatal viral diarrhea of rotaand coronavirus etiology. Veterinaria Kubani. 2008; (3): 4–6. https://elibrary.ru/kzccol (in Russ.)
15. Mischenko V. A., Mischenko A. V., Nikeshina T. B., Brovko Yu. V., Kushlubaeva A. I. Bovine nebovirus infection (review). Veterinary Science Today. 2023; 12 (4): 278–283. https://doi.org/10.29326/2304-196X-2023-12-4-278-283
16. Mischenko V. А., Mischenko A. V., Nikeshina T. B., Brovko Yu. V., Kushlubaeva А. I. Torovirus infection in animals: a review. Veterinary Science Today. 2023; 12 (2): 133–139. https://doi.org/10.29326/2304-196X-2023-12-2-133-139
17. Mishchenko V. A., Mishchenko A. V., Yashin R. V., Lysenko A. A., Krivonos R. A., Chernykh O. Yu. Features of cobuvirus infection of farm animals. Veterinaria Kubani. 2021; (5): 3–6. https://doi.org/10.33861/2071-8020-2021-5-3-6 (in Russ.)
18. Palombieri A., Fruci P., Di Profio F., Sarchese V., Robetto S., Martella V., Di Martino B. Detection and characterization of bopiviruses in domestic and wild ruminants. Transboundary and Emerging Diseases. 2022; 69 (6): 3972–3978. https://doi.org/10.1111/tbed.14676
19. Woode G. N., Bridger J. C. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. Journal of Medical Microbiology. 1978; 11 (4): 441–452. https://doi.org/10.1099/00222615-114-441
20. Bridger J. C., Hall G. A., Brown J. F. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infection and Immunity. 1984; 43 (1): 133–138. https://doi.org/10.1128/iai.43.1.133-138.1984
21. Sharp C. P., Gregory W. F., Mason C., Bronsvoort B. M., Beard P. M. High prevalence and diversity of bovine astroviruses in the faeces of healthy and diarrhoeic calves in South West Scotland. Veterinary Microbiology. 2015; 178 (1–2): 70–76. https://doi.org/10.1016/j.vetmic.2015.05.002
22. Podkolzin A. T., Konovalova T. A., Yakovenko M. L., Braslavskaya S. I., Shipulin G. A. Astroviral infection in Russian Federation. Problems of Virology. 2013; 58 (3): 32–37. https://virusjour.crie.ru/jour/article/view/12229 (in Russ.)
23. Finkbeiner S. R., Allred A. F., Tarr P. I., Klein E. J., Kirkwood C. D., Wang D. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathogens. 2008; 4 (2):e1000011. https://doi.org/10.1371/journal.ppat.1000011
24. Finkbeiner S. R., Li Y., Ruone S., Conrardy C., Gregoricus N., Toney D., et al. Identification of a novel astrovirus (astrovirus VA1) associated with an outbreak of acute gastroenteritis. Journal of Virology. 2009; 83 (20): 10836–10839. https://doi.org/10.1128/jvi.00998-09
25. De Benedictis P., Schultz-Cherry S., Burnham A., Cattoli G. Astrovirus infections in humans and animals – molecular biology, genetic diversity, and interspecies transmissions. Infection, Genetics and Evolution. 2011; 11 (7): 1529–1544. https://doi.org/10.1016/j.meegid.2011.07.024
26. Bosch A., Pintó R. M., Guix S. Human Astroviruses. Clinical Microbiology Reviews. 2014; 27 (4): 1048–1074. https://doi.org/10.1128/cmr.00013-14
27. Arias C. F., DuBois R. M. The astrovirus capsid: A review. Viruses. 2017; 9 (1):15. https://doi.org/10.3390/v9010015
28. Wohlgemuth N., Honce R., Schultz-Cherry S. Astrovirus evolution and emergence. Infection, Genetics and Evolution. 2019; 69: 30–37. https://doi.org/10.1016/j.meegid.2019.01.009
29. Family Astroviridae. In: Payne S. Viruses: From Understanding to Investigation. Academic Press; 2017; Chapter 14: 125–128. https://doi.org/10.1016/B978-0-12-803109-4.00014-3
30. Zaccaria G., Lorusso A., Hierweger M. M., Malatesta D., Defourny S. V., Ruggeri F., et al. Detection of astrovirus in a cow with neurological signs by nanopore technology, Italy. Viruses. 2020; 12 (5):530. https://doi.org/10.3390/v12050530
31. Bosch A., Guix S., Krishna N. K., Méndez E., Monroe S. S., Pantin-Jackwood M., Schultz-Cherry S. Family: Astroviridae. In: Virus Taxonomy: Classification and Nomenclature of Viruses. Ninth report of the International Committee on Taxonomy of Viruses. Eds. A. M. Q. King, M. J. Adams, E. B. Carstens, E. J. Lefkowitz. Academic Press; 2012; 953–959.
32. Donato C., Vijaykrishna D. The broad host range and genetic diversity of mammalian and avian astroviruses. Viruses. 2017; 9 (5):102. https://doi.org/10.3390/v9050102
33. Woo P. C. Y., Lau S. K. P., Teng J. L. L., Tsang A. K. L., Joseph S., Xie J., et al. A novel astrovirus from dromedaries in the Middle East. Journal of General Virology. 2015; 96 (9): 2697–2707. https://doi.org/10.1099/jgv.0.000233
34. Alfred N., Liu H., Li M. L., Hong S. F., Tang H. B., Wei Z. Z., et al. Molecular epidemiology and phylogenetic analysis of diverse bovine astroviruses associated with diarrhea in cattle and water buffalo calves in China. Journal of Veterinary Medical Science. 2015; 77 (6): 643–651. https://doi.org/10.1292/jvms.14-0252
35. Nagai M., Omatsu T., Aoki H., Otomaru K., Uto T., Koizumi M., et al. Full genome analysis of bovine astrovirus from fecal samples of cattle in Japan: identification of possible interspecies transmission of bovine astrovirus. Archives of Virology. 2015; 160 (10): 2491–2501. https://doi.org/10.1007/s00705-015-2543-7
36. Oem J. K., An D. J. Phylogenetic analysis of bovine astrovirus in Korean cattle. Virus Genes. 2014; 48 (2): 372–375. https://doi.org/10.1007/s11262-013-1013-0
37. Martella V., Catella C., Capozza P., Diakoudi G., Camero M., Lanave G., et al. Identification of astroviruses in bovine and buffalo calves with enteritis. Research in Veterinary Science. 2020; 131: 59–68. https://doi.org/10.1016/j.rvsc.2020.04.010
38. Mendenhall I. H., Smith G. J., Vijaykrishna D. Ecological drivers of virus evolution: Astrovirus as a case study. Journal of Virology. 2015; 89 (14): 6978–6981. https://doi.org/10.1128/jvi.02971-14
39. Reuter G., Nemes C., Boros A., Kapusinszky B., Delwart E., Pankovics P. Astrovirus in wild boars (Sus scrofa) in Hungary. Archives of Virology. 2012; 157 (6): 1143–1147. https://doi.org/10.1007/s00705-012-1272-4
40. Rivera R., Nollens H. H., Venn-Watson S., Gulland F. M., Wellehan J. F. Jr. Characterization of phylogenetically diverse astroviruses of marine mammals. Journal of General Virology. 2010; 91 (1): 166–173. https://doi.org/10.1099/vir.0.015222-0
41. Roach S. N., Langlois R. A. Intraand cross-species transmission of astroviruses. Viruses. 2021; 13 (6):1127. https://doi.org/10.3390/v13061127
42. Smits S. L., van Leeuwen M., Kuiken T., Hammer A. S., Simon J. H., Osterhaus A. D. Identification and characterization of deer astroviruses. Journal of General Virology. 2010; 91 (11): 2719–2722. https://doi.org/10.1099/vir.0.024067-0
43. Wang L., Shen H., Zheng Y., Schumacher L., Li G. Astrovirus in whitetailed deer, United States, 2018. Emerging Infectious Diseases. 2020; 26 (2): 374–376. https://doi.org/10.3201/eid2602.190878
44. Woode G. N., Pohlenz J. F., Gourley N. E., Fagerland J. A. Astrovirus and Breda virus infections of dome cell epithelium of bovine ileum. Journal of Clinical Microbiology. 1984; 19 (5): 623–630. https://doi.org/10.1128/jcm.19.5.623-630.1984
45. Woode G. N., Gourley N. E., Pohlenz J. F., Liebler E. M., Mathews S. L., Hutchinson M. P. Serotypes of bovine astrovirus. Journal of Clinical Microbiology. 1985; 22 (4): 668–670. https://doi.org/10.1128/jcm.22.4.668670.1985
46. Zhu Q., Li B., Sun D. Bovine astrovirus – A comprehensive review.Viruses. 2022; 14 (6):1217. https://doi.org/10.3390/v14061217
47. Candido M., Alencar A. L., Almeida-Queiroz S. R., Buzinaro M. da G., Munin F. S., de Godoy S. H., et al. Molecular detection and phylogenetic analysis of bovine astrovirus in Brazil. Archives of Virology. 2015; 160 (6): 1519–1525. https://doi.org/10.1007/s00705-015-2400-8
48. Lee T. W., Kurtz J. B. Serial propagation of astrovirus in tissue culture with the aid of trypsin. Journal of General Virology. 1981; 57 (2): 421–424. https://doi.org/10.1099/0022-1317-57-2-421
49. Caliciviridae and Astroviridae. In: Fenner’s Veterinary Virology. Ed. by N. J. MacLachlan, E. J. Dubovi. 5th ed. Academic Press; 2017; Chapter 27; 497–510. https://doi.org/10.1016/B978-0-12-800946-8.00027-1
50. Aroonprasert D., Fagerland J. A., Kelso N. E., Zheng S., Woode G. N. Cultivation and partial characterization of bovine astrovirus. Veterinary Microbiology. 1989; 19 (2): 113–125. https://doi.org/10.1016/03781135(89)90077-1
51. Reuter G., Pankovics P., Delwart E., Boros Á. Identification of a novel astrovirus in domestic sheep in Hungary. Archives of Virology. 2012; 157 (2): 323–327. https://doi.org/10.1007/s00705-011-1151-4
52. Cortez V., Meliopoulos V. A., Karlsson E. A., Hargest V., Johnson C., Schultz-Cherry S. Astrovirus biology and pathogenesis. Annual Review of Virology. 2017; 4 (1): 327–348. https://doi.org/10.1146/annurev-virology101416-041742
53. Kauer R. V., Koch M. C., Hierweger M. M., Werder S., Boujon C. L., Seuberlich T. Discovery of novel astrovirus genotype species in small ruminants. PeerJ. 2019; 7:e7338. https://doi.org/10.7717/peerj.7338
54. Zhu J., Qi M., Jiang C., Peng Y., Peng Q., Chen Y., et al. Prevalence of bovine astroviruses and their genotypes in sampled Chinese calves with and without diarrhoea. Journal of General Virology. 2021; 102 (8):001640. https://doi.org/10.1099/jgv.0.001640
55. Turan T., Işidan H. The first detection and phylogenetic analysis of bovine astrovirus from diarrheic calves in Turkey. Etlik Veteriner Mikrobiyoloji Dergisi. 2018; 29 (2): 104–110. https://doi.org/10.35864/evmd.513442
56. Mohamed F. F., Mansour S. M. G., El-Araby I. E., Mor S. K., Goyal S. M. Molecular detection of enteric viruses from diarrheic calves in Egypt. Archives of Virology. 2017; 162 (1): 129–137. https://doi.org/10.1007/s00705-016-3088-0
57. Jamnikar-Ciglenecki U., Civnik V., Kirbis A., Kuhar U. A molecular survey, whole genome sequencing and phylogenetic analysis of astroviruses from roe deer. BMC Veterinary Research. 2020; 16 (1):68. https://doi.org/10.1186/s12917-020-02289-4
58. Tzipori S., Menzies J. D., Gray E. W. Detection of astrovirus in the faeces of red deer. Veterinary Record. 1981; 108 (13):286. https://doi.org/10.1136/vr.108.13.286
59. Bouzalas I. G., Wüthrich D., Walland J., Drögemüller C., Zurbriggen A., Vandevelde M., et al. Neurotropic astrovirus in cattle with nonsuppurative encephalitis in Europe. Journal of Clinical Microbiology. 2014; 52 (9): 3318– 3324. https://doi.org/10.1128/jcm.01195-14
60. Schlottau K., Schulze C., Bilk S., Hanke D., Höper D., Beer M., Hoffmann B. Detection of a novel bovine astrovirus in a cow with encephalitis. Transboundary and Emerging Diseases. 2016; 63 (3): 253–259. https://doi.org/10.1111/tbed.12493
61. Spinato M. T., Vince A., Cai H., Ojkic D. Identification of bovine astrovirus in cases of bovine non-suppurative encephalitis in eastern Canada. The Canadian Veterinary Journal. 2017; 58 (6): 607–609. https://pubmed.ncbi.nlm.nih.gov/28588335
62. Wildi N., Seuberlich T. Neurotropic astroviruses in animals. Viruses. 2021; 13 (7):1201. https://doi.org/10.3390/v13071201
63. Nelsen A., Knudsen D., Hause B. M. Identification of a novel astrovirus associated with bovine respiratory disease. Transboundaryand Emerging Diseases. 2023; 2023:8512021. https://doi.org/10.1155/2023/8512021
64. Ng T. F., Kondov N. O., Deng X., Van Eenennaam A., Neibergs H. L., Delwart E. A metagenomics and case-control study to identify viruses associated with bovine respiratory disease. Journal of Virology. 2015; 89 (10): 5340–5349. https://doi.org/10.1128/jvi.00064-15
65. Wang D., Gao H., Zhao L., Lv C., Dou W., Zhang X., et al. Detection of the dominant pathogens in diarrheal calves of Ningxia, China in 2021–2022. Frontiers in Veterinary Science. 2023; 10:1155061. https://doi.org/10.3389/fvets.2023.1155061
66. Pérot P., Lecuit M., Eloit M. Astrovirus diagnostics. Viruses. 2017; 9 (1):10. https://doi.org/10.3390/v9010010
About the Authors
V. A. MischenkoRussian Federation
Vladimir A. Mischenko, Dr. Sci. (Veterinary Medicine), Professor, Chief Researcher, Laboratory for Biotechnologies and Viral Product Construction
Yur’evets, Vladimir 600901, Russia
A. V. Mischenko
Russian Federation
Alexey V. Mischenko, Dr. Sci. (Veterinary Medicine), Chief Researcher
24/1 Ryazansky prospekt, Moscow 109428, Russia
Т. B. Nikeshina
Russian Federation
Tatiana B. Nikeshina, Cand. Sci. (Biology), Head of Sector, Education and Scientific Support Department
Yur’evets, Vladimir 600901, Russia
О. N. Petrova
Russian Federation
Olga N. Petrova, Cand. Sci. (Biology), Deputy Head of the Sector, Information and Analysis Centre
Yur’evets, Vladimir 600901, Russia
Review
For citations:
Mischenko V.A., Mischenko A.V., Nikeshina Т.B., Petrova О.N. Astrovirus infection in animals (literature review). Veterinary Science Today. 2024;13(4):322-329. https://doi.org/10.29326/2304-196X-2024-13-4-322-329



































