Scroll to:
Gut microbiota and bacterial associations in monkeys with gastrointestinal diseases in the setting of helminth infestation
https://doi.org/10.29326/2304-196X-2024-13-2-154-163
Abstract
One of the topical issues of current primatology is spontaneous pathology in monkeys, primarily gastrointestinal infections, which are the leading ones in the morbidity and mortality patterns of the animals raised in captivity. Gastrointestinal pathology in monkeys involves complicated infectious processes, most often of associative type, with the formation of various bacterial and parasitic associations. The study demonstrates the results of gastrointestinal disease and helminth infestation monitoring as well as of the microbial flora spectrum analysis in monkeys in 2017–2022. Mortality of monkeys due to gastrointestinal diseases in the specified period amounted to 60.5%. The postmortem study demonstrated that the leading position in this pathology pattern in monkeys was taken by gastroenterocolitis (62.5%), with dominated chronic atrophic gastroenterocolitis in the acute phase (53.9%). The analysis of the six-year trend in animal mortality showed that the percentage of gastrointestinal diseases remained approximately at the same level every year. Helminth infestations were detected in 22.0% of the diseased animals and in 30.2% of the dead ones. Trichocephalus trichiurus was found in 93.3% of the diseased and in 99.7% of the dead monkeys, Strongyloides sp. – in 12.2% of the diseased and in 3.3% of the dead animals. Helminths were detected as mono- and less often as mixed infestations. In the isolated microflora, the top position was taken by the representatives of genus Proteus. The percentage of pathogenic enterobacteria detections was low, and Shigella flexneri was the leader among them. In monkeys that died from gastrointestinal diseases without parasitic infestation, the pathogenic enterobacteria detection rate was 2 times higher than in the infested animals. The microorganisms were isolated as monocultures and in associations. The microorganisms were isolated as monocultures and in associations Proteus spp. were detected more often. Gastrointestinal diseases of helminth-bacterial etiology in monkeys require complex therapy of the animals.
Keywords
For citations:
Kalashnikova V.A., Egorova T.P., Demerchyan A.V., Polyakova V.I., Lenshina Y.I., Ilyazyants D.A., Arshba I.M. Gut microbiota and bacterial associations in monkeys with gastrointestinal diseases in the setting of helminth infestation. Veterinary Science Today. 2024;13(2):154-163. https://doi.org/10.29326/2304-196X-2024-13-2-154-163
INTRODUCTION
Gut microbiota condition is one of the important factors of animal and human health. Microorganisms enter the digestive tract immediately after birth and play an important role in the animal’s life. The basis of the normal gut flora is known to consist of bifidobacteria and lactobacilli, Escherichia coli with normal fermentation, enterococci. Studies show that changes in the quantitative and qualitative composition of the gut microbiota lead to impaired intestinal function and gastrointestinal (GI) diseases [1][2][3][4]. Etiological agents of intestinal infections can be bacteria, viruses, protozoa, helminths, fungi. However, not only microbial concentration plays a significant role in the development of the intestinal diseases, but also associations of different types of microorganisms in which they enter into symbiosis or antagonism and express pathogenicity factors [5][6]. Helminths also change the quantitative and qualitative composition of the gut microbiota, forming microparasite community [7][8][9].
Monkeys are anatomically and physiologically similar to humans, and show natural susceptibility to many infectious diseases [10][11][12]. Observation results demonstrate that monkeys, both in their natural habitat and in captivity, suffer from various diseases typical for other animals and humans. In the Adler apery of the Research Institute of Medical Primatology (now Kurchatov Complex of Medical Primatology, National Research Centre “Kurchatov Institute”) monkeys of different species are kept. According to our annual data, mortality of monkeys in more than half the cases is due to gastrointestinal diseases [13][14]. Intestinal diseases of monkeys are complex infectious processes, most often of an associative type, with the formation of various bacteria and parasite combinations [15][16]. Intestinal parasites infect various species of monkeys both in the wild and in captivity, causing serious diseases of the digestive tract and in some cases leading to animal death [17][18][19][20]. According to foreign publications, nematodes are the most common helminths in monkeys kept in zoos, and they can be transmitted to humans [21][22]. In the foreign research publications, there is data about intestinal parasites isolated from primates kept in zoos and reserves or from free-living ones, but these data refer to particular species and small groups of monkeys [23][24][25]. Moreover, these papers describe only the results of parasitology research.
The relevance of the presented work is determined by the fact that GI diseases are the major cause of the monkeys’ mortality in the apery. At the same time, intestinal parasitic infections and bacterial associations that form intestinal diseases in monkeys are still an understudied problem. Our previous studies demonstrated the circulation of protozoa and helminths in the monkeys in the apery and provided some data on the concomitant microbiota [15][16]. In this paper, we would like to focus on the gut microbiota of nematode-infested monkeys and on the features of bacterial associations during its formation.
The novelty of the work lies in the fact that our study was the first to report on the microbial landscape and bacterial associations in the setting of the invasion by intestinal parasites Trichocephalus trichiurus and Strongyloides sp. in non-human primates kept in captivity.
The aim of the study was to examine the structure of the gut microbiota of monkeys with intestinal diseases who died due to GI pathology in the setting of helminth infestation.
MATERIALS AND METHODS
The study object included 2,386 monkeys of six species of both sexes aged from 10 days to 35 years, which were kept in the apery. This number included 409 monkeys affected with intestinal diseases and 1,977 dead monkeys (Tables 1, 2).
Table 1
Characteristics of tested monkeys (2017–2022)
|
Monkey species |
Diseased |
Dead |
Total |
|
Rhesus macaque (Macaca mulatta) |
172 |
731 |
903 |
|
Crab-eating macaque (Macaca fascicularis) |
152 |
514 |
666 |
|
Southern pig-tailed macaque (Macaca nemestrina) |
41 |
45 |
86 |
|
Green monkey (Chlorocebus sabaeus) |
14 |
79 |
93 |
|
Anubis baboon (Papio anubis) |
12 |
170 |
182 |
|
Hamadryas baboon (Papio hamadryas) |
18 |
438 |
456 |
|
Total |
409 |
1,977 |
2,386 |
Table 2
Number of tested dead monkeys (2017–2022)
|
Monkey species |
Number of monkeys |
Total |
|||||
|
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
||
|
Rhesus macaque |
117 |
204 |
115 |
105 |
124 |
66 |
731 |
|
Crab-eating macaque |
82 |
92 |
89 |
89 |
90 |
72 |
514 |
|
Southern pig-tailed macaque |
6 |
5 |
6 |
13 |
8 |
7 |
45 |
|
Green monkey |
11 |
20 |
17 |
17 |
3 |
11 |
79 |
|
Anubis baboon |
19 |
32 |
43 |
17 |
45 |
14 |
170 |
|
Hamadryas baboon |
57 |
55 |
75 |
66 |
105 |
80 |
438 |
|
Total |
292 |
408 |
345 |
307 |
375 |
250 |
1,977 |
The test samples included feces collected by a rectal smear from live monkeys, in case of dead animals they included the contents of three parts of the intestine (small intestine, caecum, rectum).
Bacteriological, biochemical and microscopic tests were carried out using routine practical methods1. The test material was aseptically collected and delivered to the laboratory, where initial inoculation on the diagnostic nutrient media was performed: Endo agar, Salmonella-Shigella agar, 5% blood agar, salt egg yolk agar, Yersinia selective agar, chromogenic Candida agar. The inoculates were cultivated in the thermostat at 37 °C for 24 hours, dishes with Yersinia selective agar – at 28 °C for 48 hours, dishes with Candida agar – at 24 °C for 48 hours. Isolation of pure cultures and their further identification were carried out according to generally accepted standards: examination of morphological and tinctorial properties (Gram staining of smears), hemolytic and lecithinase activity, and examination of the biochemical properties. The VITEK®2 Compact system (bioMérieux, France) was also used to determine the enterobacteria species. Slide agglutination method with specific sera was used for determination of the serovars of the isolated Shigella, Salmonella, Yersinia strains.
Parasite tests. Conventional microscopy procedure of native feces preparations was used to detect parasite infestation2. To identify helminth eggs, a small amount of feces from different places of the test portion was ground on a slide in a drop of 50% glycerol solution until a uniform transparent smear formation, covered with a coverslip and subjected to microscopy at 10 × 10 and 10 × 40 magnification. The extensity of helminth infestation was determined by the number of infected animals to the total number of the tested ones.
In the study of postmortem material, both microscopy of native preparations and macroscopic examination of the contents of the large intestine were used, as a result of which adult helminths were detected.
Statistical data processing and calculations were carried out using GraphPad Prism 8 software. To assess the significance of the differences in the frequency of helminth and bacteria detections in different monkey species in individual test groups the χ² goodness-of-fit test was used. All differences were interpreted as significant at p < 0.05. The χ² test-for-trend was used to determine changes in frequency indicators depending on the test year. Fisher’s exact test was used to determine the statistical significance between helminth infestation extensity and monkey species.
RESULTS AND DISCUSSION
From January 2017 to December 2022, 1,977 dead monkeys were tested; 1,196 (60.5%) of them demonstrated GI lesions during the necropsy (Table 3).
Table 3
Characteristics of tested dead monkeys (2017–2022)
|
Monkey species |
Dead |
Total |
|
|
with GI lesions |
without GI lesions |
||
|
Rhesus macaque |
491 |
240 |
731 |
|
Crab-eating macaque |
289 |
225 |
514 |
|
Southern pig-tailed macaque |
21 |
24 |
45 |
|
Green monkey |
52 |
27 |
79 |
|
Anubis baboon |
101 |
69 |
170 |
|
Hamadryas baboon |
242 |
196 |
438 |
|
Total |
1,196 |
781 |
1,977 |
Gastrointestinal diseases were often accompanied with pneumonia, signs of cachexia, exicosis, and dystrophy of internal organs. Analysis of the animal mortality trend over 6 years demonstrated that the percentage of GI diseases remained approximately at the same level every year. As can be seen in Table 4, there was a tendency for a slight decrease in the number of monkeys died of GI diseases in 2022.
Table 4
Trend in the monkeys’ mortality due to GI diseases, 2017–2022
|
Monkey species |
Number of dead / % |
Trend test* |
Total / % |
|||||
|
2017 (n = 292) |
2018 (n = 408) |
2019 (n = 345) |
2020 (n = 307) |
2021 (n = 375) |
2022 (n = 250) |
|||
|
Rhesus macaque (n = 731) |
77/65.8 |
140/68.6 |
79/68.7 |
75/71.4 |
85/68.5 |
35/53.0 |
< 0.0001 (↑↓) |
491/67.2 |
|
Crab-eating macaque (n = 514) |
50/61.0 |
64/69.6 |
44/49.4 |
46/51.7 |
46/51.1 |
39/54.2 |
0.3145 |
289/56.2 |
|
Southern pig-tailed macaque (n = 45) |
5/83.3 |
3/60.0 |
3/50.0 |
5/38.5 |
3/37.5 |
2/28.6 |
0.5544 |
21/46.7 |
|
Green monkey (n = 79) |
9/81.8 |
8/40.0 |
11/64.7 |
15/88.2 |
2/66.7 |
7/63.6 |
0.5575 |
52/65.8 |
|
Anubis baboon (n = 170) |
10/52.6 |
12/37.5 |
25/58.1 |
12/70.6 |
34/75.6 |
8/57.1 |
0.0429 (↑↓) |
101/59.4 |
|
Hamadryas baboon (n = 438) |
26/45.6 |
27/49.1 |
39/52.0 |
28/42.4 |
75/71.4 |
47/58.8 |
< 0.0001 (↑↓) |
242/55.3 |
|
Total |
177/60.6 |
254/62.3 |
201/58.3 |
181/59.0 |
245/65.3 |
138/55.2 |
– |
1,196/60.5 |
* p < 0.05 (χ² criterion – statistical difference of detections relative to monkey species).
Arrows show the trend of changes in detection frequency over the years upon statistical significance of the test.
Postmortem examination of dead monkeys with GI lesions showed that in 35.3% of cases the GI lesions were in the form of enterocolitis (n = 422), in 62.5% – gastroenterocolitis (n = 748) and in 0.6% – gastritis (n = 7). Furthermore, in 0.6% of cases, the intestinal lesions were associated with infectious pathology, i.e. yersiniosis (n = 4), pseudotuberculosis (n = 3). Malignant neoplasms were reported in 1.0% of monkeys: gastric adenocarcinoma (n = 3), intestinal adenocarcinoma (n = 9). According to the data obtained, the GI diseases included dominating chronic atrophic gastroenterocolitis in the acute stage (53.9%), as well as chronic forms of enterocolitis. In case of stomach lesions, only chronic atrophic gastritis was reported in monkeys (Table 5).
Table 5
GI diseases and lesions in monkeys (2017–2022)
|
GI diseases |
Number of animals / % |
Lesions, number / % |
|||
|
acute |
chronic atrophic |
chronic with complications |
CAGE (exacerbation) |
||
|
Enterocolitis |
422/35.3 |
25/5.9 |
368/87.2 |
29/6.9 |
– |
|
Gastroenterocolitis |
748/62.5 |
25/3.3 |
69/9.2 |
9/1.2 |
645/86.2 |
|
Gastritis |
7/0.6 |
0 |
7 |
0 |
– |
|
Infectious pathology |
7/0.6 |
– |
– |
– |
– |
|
Malignant neoplasms |
12/1.0 |
– |
– |
– |
– |
|
Total |
1,196/100 |
50/4.2 |
444/37.1 |
38/3.2 |
645/53.9 |
CAGE – chronic atrophic gastroenterocolitis.
As a result of parasite tests, helminth infestation was detected in 22.0% of the diseased monkeys and in 30.2% of the dead ones (Table 6). Two types of intestinal parasites were detected – Trichocephalus trichiurus and Strongyloides sp. The detection frequency of Trichocephalus trichiurus was 93.3% in the diseased monkeys (n = 84) and 99.7% in dead ones (n = 360). Strongyloides sp. were detected in 11 (12.2%) diseased and 12 (3.3%) dead animals. It was established that Strongyloides sp. mono-infestations were detected in 6 (6.7%) diseased and 1 (0.3%) dead monkeys, in other cases the helminths were detected as part of mixed infestations.
Table 6
Helminth infestation extensity in monkeys (2017–2022)
|
Monkey species |
Diseased, infested / % |
Dead monkeys with GI lesions, infested / % |
p < 0.05 |
|
Rhesus macaque |
23/13.4 |
72/14.7 |
0.2497 |
|
Crab-eating macaque |
14/9.2 |
10/3.5 |
< 0.0001 |
|
Southern pig-tailed macaque |
23/56.1 |
9/42.9 |
< 0.0001 |
|
Green monkey |
9/64.3 |
16/30.8 |
0.0662 |
|
Anubis baboon |
8/66.7 |
73/72.3 |
0.0134 |
|
Hamadryas baboon |
13/72.2 |
181/74.8 |
< 0.0001 |
|
Total |
90/22.0 |
361/30.2 |
Table 6 demonstrates that in the crab-eating macaques with intestinal diseases the helminth detection frequency was higher than in the dead ones. As for Anubis baboons, Trichocephalus trichiurus was detected somewhat more often in the intestines of the dead animals. The frequency of infection with these parasites in the diseased and dead rhesus monkeys was almost the same. The same situation was reported in hamadryas baboons. Over the 6-year period, a small number of southern pig-tailed macaques and green monkeys were examined, however, the resulted data demonstrated that helminths were more often detected in the diseased animals of these species than in the dead ones. It was noted that Strongyloides sp. were found only in 3 species: rhesus macaques, green monkeys and hamadryas baboons. Thus, the results of the work showed that Trichocephalus trichiurus often infected non-human primates, and this coincided with the data of the foreign studies [26][27][28].
In 2017–2022, as a result of bacteriological tests of the feces of the diseased monkeys and intestinal contents of the dead ones, 1,468 microorganisms were detected and isolated gut microbiota was characterized by species diversity; 242 microorganisms were isolated from the feces of the diseased monkeys. The proportion of gram-negative gut microbiota was 80.6% (n = 195), gram-positive – 18.6% (n = 45), yeast-like fungi – 0.8% (n = 2). Representatives of the Enterobacteriaceae family were found in 43.5% of the diseased animals with pathogenic enterobacteria isolated in 1.9% of monkeys (n = 8) and opportunistic ones – in 41.6% (n = 170). Coccal microorganisms detected in 9.1% of animals included Staphylococcus spp. (6.6%), hemolytic Enterococcus spp. (2.2%), gram-positive diplococci (0.3%); 1,226 microorganisms were isolated from the dead monkeys, of which 95.4 % belonged to the gram-negative gut microbiota (n = 1,170), 2.8% belonged to the gram-positive gut microbiota (n = 34); the proportion of yeast-like fungi amounted to 1.8% (n = 22). In 96.0% of the dead animals, enterobacteria prevailed in the isolated gut microbiota (n = 1,148), of which the proportion of pathogenic ones amounted to 7.2% (n = 83), and opportunistic – to 92.8% (n = 1,065). Gram-positive cocci were found in the intestines of 1.5% of the dead monkeys (n = 18), while Staphylococcus spp. was detected in 0.7% of the animals, hemolytic Enterococcus spp. in 0.3%, and gram-positive diplococcus in 0.5%. Pathogenic and opportunistic gut microbiota was not detected in 282 dead and 220 diseased monkeys (23.6 and 53.8%, respectively). No bacterial growth was reported after inoculation of the samples from 3 diseased monkeys onto the nutrient media (0.7%).
The study results demonstrated that representatives of the genus Proteus dominated in the gut microbiota, their detection was three times more likely in the dead animals than in the diseased ones (55.0 and 16.4%, respectively).
Klebsiella spp., Staphylococcus spp., hemolytic Enterococcus spp., Enterobacter spp., Pseudomonas aeruginosa were more often isolated from the diseased animals. Providencia spp., Enterobacter spp., Shigella flexneri, Morganella morganii, Klebsiella spp., Citrobacter spp. were more often detected in the dead animals with GI diseases (Fig. 1).
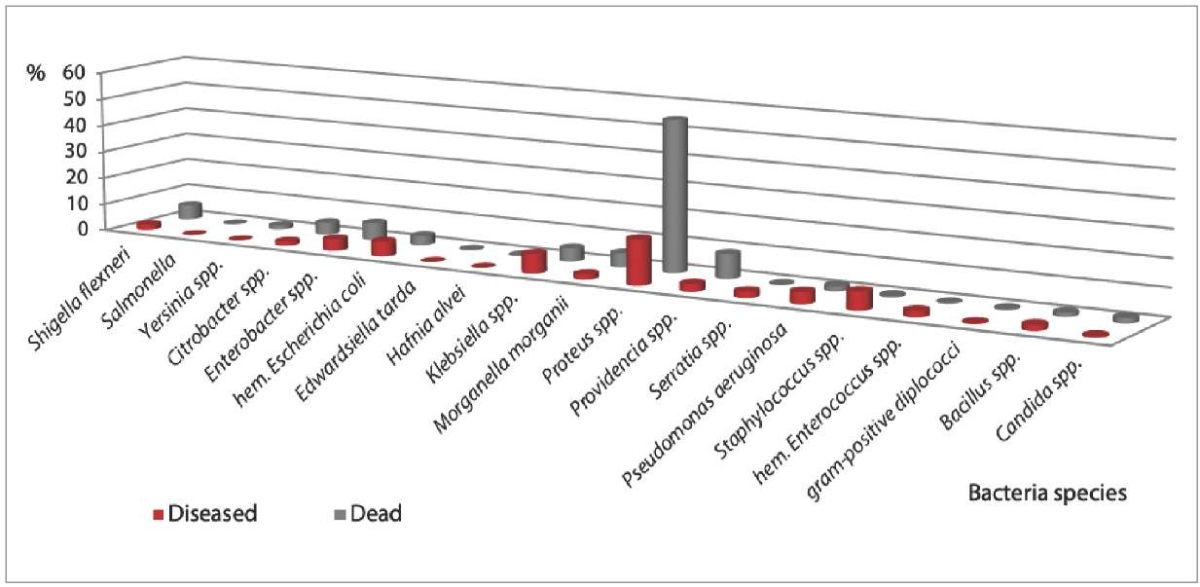
Fig. 1. Gut microbiota composition (or species diversity) in tested monkeys (2017–2022)
Analysis of the bacterial gut microbiota composition of monkeys indicated (Fig. 2) that non-helminth-infested animals with the highest frequency demonstrated Klebsiella spp. (7.8% – in the diseased animals, 5.5% – in the dead animals) and Enterobacter spp. (5.0% – in the diseased animals, 7.6% – in the dead animals). At the same time, Staphylococcus spp. (6.9%), hemolytic Escherichia coli (6.0%), Pseudomonas aeruginosa (4.4%) were more often isolated from the diseased monkeys without parasite infestation, and Providencia spp. (5.6%), Citrobacter spp. (5.2%), Morganella morganii (4.4%) were most frequent in the dead parasite-free animals. As for helminth infested animals, Staphylococcus spp. (5.6%), Klebsiella spp. (4.5%), Bacillus spp. (4.5%) were most often detected in the diseased monkeys, and Providencia spp. (16.9%), Morganella morganii (6.7%) were found in the dead ones.
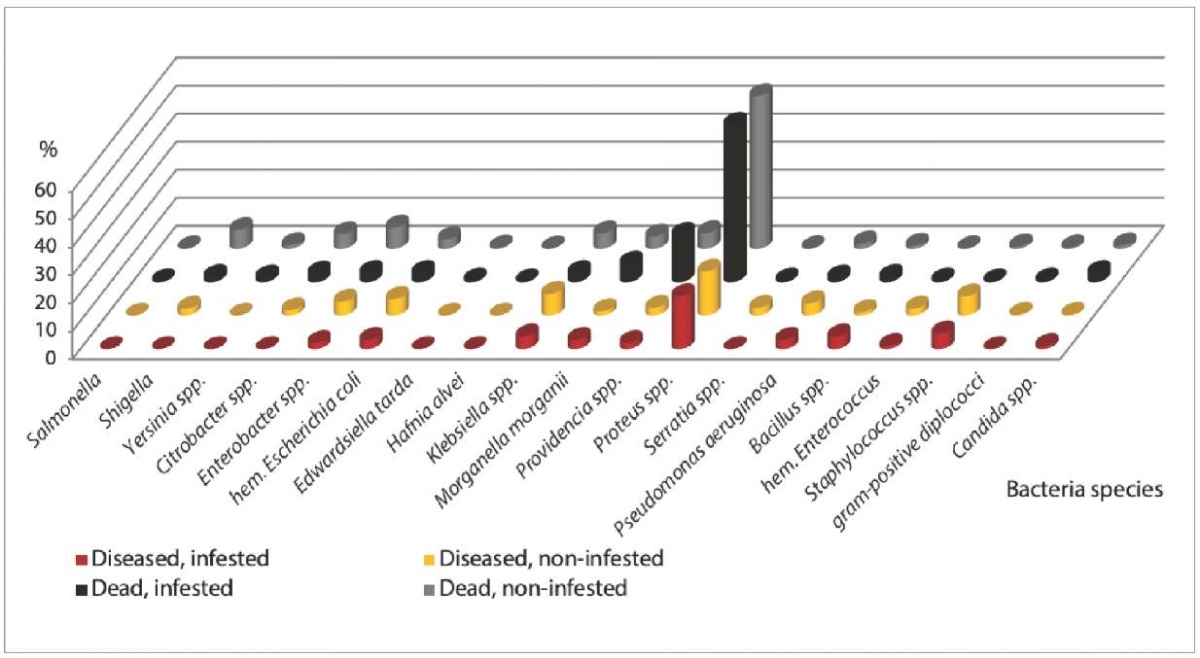
Fig. 2. Effect of helminth infestation on the frequency of detection
of the pathogenic and opportunistic bacteria in monkeys (2017–2022)
It was noted that pathogenic enterobacteria were not detected in the helminth infested diseased animals, while in the helminth-free monkeys Shigella flexneri were isolated in 2.5% of cases (n = 8). In dead monkeys without helminth infestation, the frequency of the detection of pathogenic enterobacteria (Shigella flexneri, rare Salmonella serovars, Yersinia spp.) was 2 times higher than in the infested animals – 8.3% (n = 69) and 3.9% (n = 14), respectively. As a result, we assume that presence of the intestinal helminths can reduce the number of bacterial pathogens, occupying their niche in the gut biocenosis.
When analyzing the trend in the frequency of isolation of gut microbiota bacteria over six years, a consistently high annual percentage of Proteus spp. detections was established (Table 7).
Table 7
Frequency of microbiota detection in GI diseased and dead monkeys (2017–2022)
|
Detected microorganisms |
Quantity / % |
Trend test* |
Total / % |
|||||
|
2017 (n = 177) |
2018 (n = 254) |
2019 (n = 201) |
2020 (n = 181) |
2021 (n = 245) |
2022 (n = 138) |
|||
|
Citrobacter spp. |
8/4.5 |
13/5.1 |
17/8.5 |
13/7.2 |
3/1.2 |
6/4.3 |
0.1953 |
60/5.0 |
|
Enterobacter spp. |
7/4.0 |
15/5.9 |
14/7.0 |
17/9.4 |
20/8.2 |
20/14.5 |
0.0007 (↑) |
93/7.8 |
|
Hemolytic Escherichia coli |
9/5.1 |
8/3.1 |
8/4.0 |
13/7.2 |
14/5.7 |
12/8.7 |
0.0445 (↑↓) |
64/5.4 |
|
Edwardsiella tarda |
2/1.1 |
0 |
0 |
1/0.6 |
2/0.8 |
0 |
0.7845 |
5/0.4 |
|
Hafnia alvei |
1/0.6 |
0 |
1/0.5 |
0 |
0 |
0 |
0.9010 |
2/0.2 |
|
Klebsiella spp. |
11/6.2 |
13/5.1 |
27/13.4 |
24/13.3 |
4/1.6 |
8/5.8 |
0.3492 |
87/7.3 |
|
Morganella morganii |
6/3.4 |
15/5.9 |
21/10.4 |
9/5.0 |
13/5.3 |
5/3.6 |
0.7308 |
69/5.8 |
|
Proteus spp. |
86/48.6 |
168/66.1 |
121/60.2 |
103/56.9 |
171/69.8 |
77/55.8 |
0.0914 |
726/60.7 |
|
Providencia spp. |
7/4.0 |
17/6.7 |
24/11.9 |
19/10.5 |
28/11.4 |
22/16.0 |
0.0002 (↑↓) |
117/9.8 |
|
Salmonella of rare serovars |
2/1.1 |
1/0.4 |
0 |
0 |
0 |
0 |
0.0279 (↓) |
3/0.3 |
|
Serratia spp. |
1/0.6 |
1/0.4 |
1/0.5 |
0 |
0 |
10/7.2 |
0.0002 (↓↑) |
13/1.1 |
|
Shigella flexneri |
0 |
30/11.8 |
17/8.5 |
9/5.0 |
9/3.7 |
6/4.3 |
0.3172 |
71/5.9 |
|
Yersinia spp. |
0 |
3/1.2 |
2/1.0 |
6/3.3 |
0 |
6/4.3 |
0.0330 (↓↑) |
17/1.4 |
|
Pseudomonas aeruginosa |
3/1.7 |
11/4.3 |
18/9.0 |
5/2.8 |
2/0.8 |
0 |
0.0186 (↑↓) |
39/3.3 |
|
Bacillus spp. |
0 |
6/2.4 |
10/5.0 |
3/1.7 |
0 |
5/3.6 |
0.7586 |
24/2.0 |
|
Hemolytic Enterococcus |
7/4.0 |
1/0.4 |
0 |
1/0.6 |
2/0.8 |
2/1.4 |
0.1159 |
13/1.1 |
|
Staphylococcus spp. |
4/2.3 |
3/1.2 |
11/5.5 |
1/0.6 |
12/4.9 |
4/2.9 |
0.2040 |
35/2.9 |
|
Gr+ diplococcus |
1/0.6 |
5/2.0 |
0 |
0 |
0 |
0 |
0.0183 (↑↓) |
6/0.5 |
|
Candida spp. |
0 |
2/0.8 |
7/3.5 |
1/0.6 |
1/0.4 |
13/9.4 |
0.0001 (↑) |
24/2.0 |
* p < 0.05 (χ 2 criterion – statistical difference of detections relative to monkey species).
Arrows show the trend of changes in detection frequency over the years
upon statistical significance of the test.
Monitoring of the isolation of pathogenic and opportunistic bacteria in monkeys during 2017–2022 showed decrease in the frequency of detections of Klebsiella spp., Morganella morganii, Shigella spp., Pseudomonas spp., hemolytic Enterococcus spp. and increase in the frequency of detections of hemolytic Escherichia coli, Enterobacter spp., Providencia spp., Serratia spp., Yersinia spp., yeast-like fungi of genus Candida. The decrease in the frequency of detections of Klebsiella spp. and Shigella flexneri might be associated with the use of Klebsiella phage and intestinal phage in the treatment of the animals. The highest percentage of detections of Citrobacter spp., Klebsiella spp., Morganella morganii, Pseudomonas aeruginosa, Staphylococcus spp., Bacillus spp., as compared with other years, was recorded in 2019.
The microorganisms were isolated both as mono-infestations and mixed infestations. Combinations of normal flora representatives with Proteus spp. were more often detected. Thus, in the diseased helminth-infested monkeys, the association of Escherichia coli + Proteus spp. was reported in 10.0% of cases, Escherichia coli + Enterococcus + Proteus spp. – in 4.5% of cases, Escherichia coli + Enterobacter spp. + Staphylococcus spp. – in 2.2% of cases. The highest percentage of simultaneous detections of Escherichia coli + Proteus spp. was also reported in the diseased helminth non-infested monkeys (9.4%). Combinations of Escherichia coli + Enterococcus spp. + Proteus spp. were reported in 4.1% of animals; bacterial associations of normal flora involving Escherichia coli + Enterococcus spp. + Klebsiella spp. and Escherichia coli + Enterococcus spp. + Staphylococcus spp. were detected in 3.5% of monkeys. Combinations of hemolytic Escherichia coli with Enterococcus spp. were reported in 2.5% of the animals, normal flora with Enterobacter spp. – in 2.2% of the cases. Associations of Escherichia coli + Serratia spp., Escherichia coli + Klebsiella spp., Escherichia coli + Enterococcus spp. + Pseudomonas aeruginosa were detected in 1.9% of the tested monkeys. The remaining variants of microbial associations were identified in rare cases.
In the dead monkeys the bacterial associations were more diverse. Thus, two-component associations were found in 53.0% of cases, three-component associations in 36.7%, four-component associations in 6.7%, five-component associations in 2.5% and six-component associations in 0.3% of cases. As in the diseased monkeys, Proteus spp. were most often detected concurrently with the normal flora in the dead monkeys (39%). Combinations involving normal flora with other opportunistic bacteria were much less common. Thus, co-growth of Escherichia coli and Enterococcus spp. with the following bacteria were observed: Providencia spp. – in 3.5% of cases, Enterobacter spp. – in 2.9% of cases, Shigella flexneri – in 2.4% of cases, Citrobacter spp. – in 2.0% of cases, Morganella morganii – in 1.8% of cases, and Klebsiella spp. – in 1.3% of cases. In rare cases, Proteus spp. were isolated from the intestines of the dead animals together with Citrobacter spp., Enterobacter spp., Yersinia spp. without normal flora. The proportion of concurrent detections of Proteus spp. + Enterococcus spp. amounted to 1.0%.
Associations of yeast-like fungi of genus Candida in the intestines of the dead monkeys with one representative of the opportunistic microbiota were detected more often than with two ones (13 and 4 cases, respectively). Incidence of Candida spp. in association with Proteus spp. (11 monkeys) was higher than with Klebsiella spp. (3 monkeys) and Pseudomonas aeruginosa (2 monkeys). Therefore, combinations of pathogenic and opportunistic bacteria with yeast-like fungi in helminth-infested monkeys can aggravate the course of GI diseases due to the simultaneous involvement of pathogenicity factors of various microorganisms and parasites in the development of the infectious process.
As for the species composition of the microbiota, the following Enterobacteriaceae species were isolated from monkeys: Citrobacter freundii, C. diversus, C. amalonaticus, Enterobacter aerogenes, E. agglomerans, E. cloacae, E. gergoviae, Klebsiella pneumoniae, K. oxytoca, K. ozaenae, Proteus vulgaris, Pr. mirabilis, Pr. penneri, Providencia stuartii, P. rettgeri, P. alcalifaciens, Serratia marcences, S. odorifera; Enterococcus: Enterococcus faecalis, E. faecium; Staphylococcus: Staphylococcus aureus, S. haemolyticus; as well as yeast-like fungi: Candida krusei, C. glabrata, C. tropicalis.
In conclusion, it can be noted that an important role in the development of GI diseases in monkeys is also played by weakened immunity due to various external factors, including stress, breaches of veterinary and sanitary, zootechnical and animal hygiene rules of feeding and keeping monkeys, which lead to normal gut microbiota disorders and opportunistic microbiota activation. Thus, GI diseases of helminth and bacterial etiology in monkeys require complex therapy. When keeping monkeys in captivity, there is a risk of parasite and pathogenic microorganism transmission to handlers due to human-animal contact. The detected Trichocephalus trichiurus and Strongyloides sp. are dangerous to humans, therefore it is necessary to comply with safety requirements when working with the diseased monkeys (regular deworming of animals, daily cleaning of cages and enclosures, and strict compliance with the personal hygiene rules). Knowledge about the parasitic and bacterial agents of spontaneous intestinal infections in monkeys is necessary for proper and safe breeding and keeping of these rare animals in captivity and for the practical use of monkeys in biomedical research.
CONCLUSIONS
The following conclusions were made based on the study results.
- 1. Trichocephalus trichiurus are prevalent in the non-humane primates kept in the apery.
- Etiology of GI diseases in monkeys involves various associations of diverse bacteria with prevailing representatives of the Enterobacteriaceae family.
- The dominant microorganisms were Proteus spp., which were isolated from 16.4% of the monkeys with GI diseases, and from 55.0% of the dead animals.
- The percentage of pathogenic enterobacteria detections was low (diseased monkeys – 1.9%, dead monkeys – 7.2%), but Shigella flexneri was the leader among them.
- In helminth non-infested monkeys, the pathogenic enterobacteria detection frequency was higher than in the infested ones.
- Associative GI diseases of helminth and bacterial etiology require complex therapy of monkeys.
- When keeping monkeys in captivity, there is a risk of parasite and pathogenic microorganism transmission to the handlers due to human-animal contact.
Contribution: Kalashnikova V. A. – selection and analysis of published literature on the subject, result interpretation, compilation of tables and diagrams, text preparation; Egorova T. P. – parasite tests, consultations on parasite test results; Demerchyan A. V. – bacteriological tests; Polyakova V. I. – bacteriological tests, statistical data processing; Lenshina Ya. I. – parasite tests; Ilyazyants D. A. – pathomorphologic examination (necropsy of monkeys, diagnosis); Arshba I. M. – bacteriological tests.
Вклад авторов: Калашникова В. А. – подбор и анализ литературных источников по теме, обработка результатов, составление таблиц и диаграмм, подготовка текста; Егорова Т. П. – проведение паразитологических исследований, консультирование по данным паразитологического исследования; Демерчян А. В. – проведение бактериологических исследований; Полякова В. И. – проведение бактериологических исследований, статистическая обработка данных; Леншина Я. И. – проведение паразитологических исследований; Ильязянц Д. А. – проведение патоморфологических исследований (вскрытие обезьян в прозектуре, постановка диагноза); Аршба И. М. – проведение бактериологических исследований.
1. Methodological guidelines for the microbiological diagnosis of the enterobacteria-induced diseases: approved by the Ministry of Health of the USSR on 17.12.1984 No. 04-723/3. https://base.garant.ru/71310616/?ysclid=lvdnbim4fh245607194
2. MUC 4.2.3145-13 Laboratory diagnostics of helminth and protozoa infestations: guidelines (approved by Chief State Medical Officer of the Russian Federation on 26 November 2013). https://docs.cntd.ru/document/1200110752?ysclid=lvdnu57iyo743363677
References
1. Denisenko O. V., Gapon M. N., Ternovskay L. N., Tverdohlebova T. I. The species composition of conditionally pathogenic microflora at the large intestine dysbacterioses in residents of Rostov-on-Don. Kuban Scientific Medical Bulletin. 2013; (1): 74–77. https://elibrary.ru/qccqbr (in Russ.)
2. Egorova S. A., Makarova M. A., Kaftyreva L. A. Opportunictic Enterobacteriacae as the cause of the acute diarrhea and gut disbiosis. Russian Journal of Infection and Immunity. 2011; 1 (2): 181–184. https://doi.org/10.15789/2220-7619-2011-2-181-184 (in Russ.)
3. Ivanova E. I., Kolesnikova L. I., Rychkova L. V., Savelkaeva M. V., Nemchenko U. M., Rakova E. B. Microecological and associative structure of intestinal biocenosis in children with functional gastrointestinal disorders. Bulletin of the East Siberian Scientific Center of the Academy of Medical Sciences. 2016; 1 (5): 22–25. https://elibrary.ru/wxbrkl (in Russ.)
4. Ivanova E. I., Popkova S. M., Rakova E. B., Nemchenko U. M., Savelkayeva M. V., Gorbunova E. L. Study of the genus Candida fungi associations with some opportunistic microorganisms in persons with functional disorders of the gastrointestinal tract. Bulletin of the East Siberian Scientific Center of the Academy of Medical Sciences. 2011; (3-1): 196–198. https://elibrary.ru/oomfaj (in Russ.)
5. Skuratovich E. G. Microbiocenosis of the gastrointestinal tract in young deer European: age dynamics for the first year of life. Problems of Productive Animal Biology. 2019; (3): 96–105. https://elibrary.ru/zpewab (in Russ.)
6. Ivanyuk V. P., Bobkovа G. N. Changes of microbiocenosis of pigs’ intestine with helminth infestation. Vestnik of the Bryansk State Agricultural Academy. 2017; (1): 19–22. https://elibrary.ru/xvktpb (in Russ.)
7. Katkov A. E., Romanova E. M. Osobennosti mikrobiotsenoza kishechnika na fone strongiloidnoi invazii = Intestinal microbiocenosis specifics in the setting of Strongyloides infestation. Vestnik of Ulyanovsk State Agricultural Academy. 2007; 2 (5): 61–66. https://elibrary.ru/rvweev (in Russ.)
8. Aslanova M. M., Zagaynova A. V., Kuznetsova K. Y., Rakova V. M., Smetanina N. V. Study of the composition of the intestinal microbiota by parasitological indicators in the population belonging to different health groups. Medical Parasitology and Parasitic Diseases. 2019; (2): 19–25. https://doi.org/10.33092/0025-8326mp2019.2.19-25 (in Russ.)
9. Lee S. C., Tang M. S., Lim Y. A. L., Choy S. H., Kurtz Z. D., Cox L. M., et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Neglected Tropical Diseases. 2014; 8 (5):e2880. https://doi.org/10.1371/journal.pntd.0002880
10. Lapin B. A., Dzhikidze E. K., Fridman E. P. Medical primatology guide. Moscow: Meditsina; 1987. 192 p. (in Russ.)
11. Blersch R., Archer C., Suleman E., Young C., Kindler D., Barrett L., Henzi S. P. Gastrointestinal parasites of vervet monkeys (Chlorocebus pygerythrus) in a high latitude, semi-arid region of South Africa. Journal of Parasitology. 2019; 105 (4): 630–637. https://doi.org/10.1645/19-19
12. Obanda V., Maingi N., Muchemi G., Ng’ang’a C. J., Angelone S., Archie E. A. Infection dynamics of gastrointestinal helminths in sympatric non-human primates, livestock and wild ruminants in Kenya. PLoS ONE. 14 (6):e0217929. https://doi.org/10.1371/journal.pone.0217929
13. Kalashnikova V. A. Detection of Campylobacter jejuni and Helicobacter pylori associated with gastrointestinal diseases of monkeys. Russian Journal of Veterinary Pathology. 2009; (4): 8–12. https://www.vetpat.ru/jour/article/view/1197 (in Russ.)
14. Ardasheliya S. N., Kalashnikova V. A., Dzhikidze E. K. Etiologic structure of bacterial intestinal infections in monkeys of Adler breeding center. Bulletin of Experimental Biology and Medicine. 2011; 151 (6): 734–737. https://doi.org/10.1007/s10517-011-1428-3
15. Egorova T. P., Arshba I. M., Demerchyan A. V., Lenshina Y. I. Species diversity and structure of parasitic-bacterial combinations in intestinal diseases of monkeys. Veterinariya. 2023; (2): 31–35. https://doi.org/10.30896/0042-4846.2023.26.2.31-35 (in Russ.)
16. Egorova T. P. Data on intestinal parasites of lower monkeys in the Adler apery. Parazitologiya. 2010; 44 (4): 343–350. https://elibrary.ru/oizsdv (in Russ.)
17. Adrus M., Zainudin R., Ahamad M., Jayasilan M.-A., Abdullah M. T. Gastrointestinal parasites of zoonotic importance observed in the wild, urban, and captive populations of non-human primates in Malaysia. Journal of Medical Primatology. 2019; 48 (1): 22–31. https://doi.org/10.1111/jmp.12389
18. Boundenga L., Ngoubangoye B., Moukodoum N., Dibakou S. E., Moussadji C., Hugot J. P. Diversity of parasites in two captive chimpanzee populations in southern Gabon. Infection, Genetics and Evolution. 2021; 91:104807. https://doi.org/10.1016/j.meegid.2021.104807
19. Islam S., Rahman M. K., Uddin M. H., Rahman M. M., Chowdhury M. N. U., Hassan M. M., et al. Prevalence and diversity of gastrointestinal parasites in free‐ranging rhesus macaques (Macaca mulatta) in different land gradients of Bangladesh. American Journal of Primatology. 2022; 84 (1):e23345. https://doi.org/10.1002/ajp.23345
20. Klyueva A. K., Egorova T. P., Deltsov A. A. Parasite fauna of Javanese macaques (Macaca fascicularis). Veterinariya, Zootekhniya i Biotekhnologiya. 2021; 11: 72–77. https://doi.org/10.36871/vet.zoo.bio.202111010 (in Russ.)
21. Vonfeld I., Prenant T., Polack B., Guillot J., Quintard B. Gastrointestinal parasites in non-human primates in zoological institutions in France. Parasite. 2022; 29:43. https://doi.org/10.1051/parasite/2022040
22. Kumar S., Sundararaj P., Kumara H. N., Pal A., Santhosh K., Vinoth S. Prevalence of gastrointestinal parasites in bonnet macaque and possible consequences of their unmanaged relocations. PLoS ONE. 2018; 13 (11):e0207495. https://doi.org/10.1371/journal.pone.0207495
23. N’da K. M., Dahourou L. D., Ndiaye P. I., Lindshield S., Gbati O. B., Traore A. Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal. Open Veterinary Journal. 2022; 12 (4): 481–488. https://doi.org/10.5455/OVJ.2022.v12.i4.9
24. Tabasshum T., Liza F. T., Rabbe M. F., Mukutmoni M., Alam M. M., Begum A. Occurrence of gastrointestinal (GI) parasites in captive Olive Baboon and Common Langur in Bangladesh. Animal Diseases. 2022; 2:4. https://doi.org/10.1186/s44149-022-00037-9
25. Petrášová J., Modrý D., Huffman M. A., Mapua M. I, Bobáková L., Mazoch V., et al. Gastrointestinal parasites of indigenous and introduced primate species of Rubondo Island National Park, Tanzania. International Journal of Primatology. 2010; 31: 920–936. https://doi.org/10.1007/s10764-010-9439-x
26. Valenta K., Twinomugisha D., Godfrey K., Liu C., Schoof V. A. M., Goldberg T. L., Chapman C. A. Comparison of gastrointestinal parasite communities in vervet monkeys. Integrative Zoology. 2017; 12 (6): 512–520. https://doi.org/10.1111/1749-4877.12270
27. Kouassi R. Y. W., McGraw S. W., Yao P. K., Abou-Bacar A., Brunet J., Pesson B., et al. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Taï National Park, Côte d’Ivoire. Parasite. 2015; 22:1. https://doi.org/10.1051/parasite/2015001
28. Tandan S., Kshetri S., Paudel S., Dhakal P., Kyes R. C., Khanal L. Prevalence of gastrointestinal helminth parasites in rhesus macaques and localresidents in the central mid-hills of Nepal. Helminthologia. 2023; 60 (4): 327–335. https://doi.org/10.2478/helm-2023-0037
About the Authors
V. A. KalashnikovaRussian Federation
Victoria A. Kalashnikova, Cand. Sci. (Biology), Leading Researcher, Laboratory of Infectious Pathology
177 Mira str., Vesyoloye, Adlersky City District, Sochi 354376, Krasnodar Krai
T. P. Egorova
Russian Federation
Tat’yana P. Egorova, Senior Researcher, Laboratory of Infectious Pathology
177 Mira str., Vesyoloye, Adlersky City District, Sochi 354376, Krasnodar Krai
A. V. Demerchyan
Russian Federation
Alvard V. Demerchyan, Researcher, Laboratory of Infectious Pathology
177 Mira str., Vesyoloye, Adlersky City District, Sochi 354376, Krasnodar Krai
V. I. Polyakova
Russian Federation
Veronika I. Polyakova, Junior Researcher, Laboratory of Infectious Pathology
177 Mira str., Vesyoloye, Adlersky City District, Sochi 354376, Krasnodar Krai
Y. I. Lenshina
Russian Federation
Yana I. Lenshina, Postgraduate Student, Research Assistant, Laboratory of Infectious Pathology
177 Mira str., Vesyoloye, Adlersky City District, Sochi 354376, Krasnodar Krai
D. A. Ilyazyants
Russian Federation
David A. Ilyazyants, Junior Researcher, Laboratory of Pathological Anatomy
177 Mira str., Vesyoloye, Adlersky City District, Sochi 354376, Krasnodar Krai
I. M. Arshba
Russian Federation
Ilona M. Arshba, Cand. Sci. (Biology), Leading Researcher, Acting Head of the Laboratory of Infectious Pathology
177 Mira str., Vesyoloye, Adlersky City District, Sochi 354376, Krasnodar Krai
Review
For citations:
Kalashnikova V.A., Egorova T.P., Demerchyan A.V., Polyakova V.I., Lenshina Y.I., Ilyazyants D.A., Arshba I.M. Gut microbiota and bacterial associations in monkeys with gastrointestinal diseases in the setting of helminth infestation. Veterinary Science Today. 2024;13(2):154-163. https://doi.org/10.29326/2304-196X-2024-13-2-154-163
JATS XML



































