Scroll to:
Development and application of ELISA test system for assessing humoral immunity against SAT2 topotype XIV foot-and-mouth disease virus
https://doi.org/10.29326/2304-196X-2025-14-3-283-293
Abstract
Introduction. Foot-and-mouth disease (FMD) is a highly contagious, economically significant disease of cloven-hoofed animals, characterized by vesicular symptoms. There are seven known foot-and-mouth disease virus (FMDV) serotypes (A, O, C, SAT 1, SAT 2, SAT 3 and Asia 1), which immunologically differ from each other. Special attention has been recently paid to FMDV of SAT2/XIV topotype (South African Territories 2) due to its rapid spread. One of the key methods of FMD control is vaccination and assessment of the susceptible animal immune status.
Objective. Development and testing of an enzyme-linked immunosorbent assay (ELISA) system based on the indirect liquid-phase blocking ELISA for the determination of antibodies to structural proteins of SAT2/XIV FMDV in order to evaluate the effectiveness of an FMD vaccine based on SAT-2/XIV/2023 FMDV antigen in the process of its production and subsequent use.
Materials and methods. The test material included experimental serum samples collected from cattle, pigs and white mice. The developed ELISA test system for assessing the level of post-vaccination antibodies against SAT2/XIV FMDV was validated through comparative testing with commercial test-kits: Test-kit for the determination of SAT 2 FMDV antibodies (Federal Centre for Animal Health, Russia) and “Solid-phase competitive ELISA for antibodies specific to FMDV serotype SAT 2”(IZSLER & The Pirbright Institute, Italy/Great Britain).
Results. The effectiveness of the proposed test system in detecting the induction of antibodies against SAT2/XIV FMDV was higher than that of other ELISA systems with the pronounced topotype specificity to the SAT2/VII FMD agent. Specific antibodies were detected in individual cattle on day 7 post vaccination. High diagnostic sensitivity (90%), specificity (98%) and accuracy (95%) ensured high degree of the ELISA results consistency with the known diagnostic status of the tested animals (k-criterion – 0.896).
Conclusion. Thus, the ELISA system for assessing humoral immunity against SAT2/XIV FMDV, which is 100% homologous with the vaccine strain and demonstrates high diagnostic parameters, is a reliable tool for assessing the quality of the SAT2/XIV FMD vaccine.
For citations:
Lugovskaya N.N., El’kina Yu.S., Shevchenko M.A., Gochmuradov Y.M., Klyukina N.D., Mikhalishin D.V., Borisov A.V. Development and application of ELISA test system for assessing humoral immunity against SAT2 topotype XIV foot-and-mouth disease virus. Veterinary Science Today. 2025;14(3):283-293. https://doi.org/10.29326/2304-196X-2025-14-3-283-293
INTRODUCTION
Foot-and-mouth disease (FMD) is a highly contagious, economically significant disease of cloven-hoofed animals, which is characterized by vesicular symptoms. There are seven known FMDV serotypes: A, O, C, SAT 1, SAT 2, SAT 3 and Asia 1, which immunologically differ from each other.
The disease causative agent in the susceptible animals is an Aphthovirus of the Picornaviridae family, which is a capsid enclosing a positive sense RNA chain. The capsid of an icosahedron structure with a sedimentation coefficient 146S, is formed of 60 copies of a protomeric subunit consisting of four structural proteins (SP): VP1–VP4. VP1–VP3 surface polypeptides carry epitopes responsible for the FMDV serotype specificity and inducing production of the virus neutralizing antibodies. The priority polypeptide is VP1, which features the major antigenic site located within the G-H loop region. The internal protein VP4 is highly conserved across the serotypes of the pathogen, and antibodies against VP4 epitopes do not confer protection against the infection [1][2].
Domesticated animals, mainly cattle, pigs, sheep, goats, buffaloes (Bubalus bubalis), as well as camels and New World camelids, are FMD susceptible. However, the FMDV is sometimes isolated from wild cloven-hoofed animals such as wild pigs, antelopes and deer. Cross-infection between wild and domestic cloven-hoofed animals is therefore possible through direct or indirect contact. In sympatric species, this is one of the most likely routes of FMDV transmission. The primary FMDV reservoir is cattle, as this species is characterized by the virus carrier phenomenon. The virus carriers are individuals in whose oropharynx the virus persists for more than four weeks after infection. In cattle, this condition usually persists for up to six months, but in some individuals it lasts for up to three years. In wild animals, the virus carrier state was proven only for African buffaloes (Syncerus caffer), some individuals of which maintained the virus for five years, and in the herd the pathogen can circulate for 24 years or more. This characteristic of cattle significantly complicates FMD control efforts, particularly in the disease endemic areas. Such areas include a number of countries in Africa, Middle East, and Asia, as well as in a limited area of South America [1], which in turn creates a serious risk of FMD spread outside the endemic territories.
The FMD control consists in timely preventive measures, such as effective epidemiological disease control; vaccination of domestic and farm animals against FMD; movement control of wild cloven-hoofed animals in border areas at risk of the pathogen introduction from the FMD-infected countries; monitoring tests to assess the immunity level of farm and domestic animals, including retrospective FMD diagnosis. Since the viruses of different serotypes induce virtually no cross-immunity, and the vaccines, which are based on strains different from the field ones, may provide weak protection even within the same serotype, the development of vaccine formulations requires meticulous strain selection to ensure maximum possible protection of the animal population against FMD in a specific region [1][2].
Recently, special attention has been paid to serotype SAT 2 FMDV, which is exotic for many regions of the world, including for Russia. The name itself, which is an English abbreviation of South African Territories, indicates the origin of this serotype. FMD outbreaks caused by serotype SAT 2 virus, whose representatives are characterized by high genetic variability and are divided into 14 topotypes (I-XIV), are periodically reported in the African continent [3]. Until recently, the FMD epidemic situation in Africa was generally associated with the members of serotype SAT 2 topotype VII (SAT2/VII), but in 2022, rapid spread of SAT 2 topotype XIV (SAT2/XIV) virus started, which resulted in its spread outside the endemic territories. FMD outbreaks caused by SAT2/XIV topotype virus were reported in 2023 in some Eurasian countries, including Iraq, Jordan, and Turkey.
Serotype SAT 2 FMDV, which circulated in Iraq in 2023, is closely related to the virus isolates recovered in Ethiopia in 2022 (SAT2/ETH/3/22 and SAT2/ETH/2/22). Both viruses were isolated from the infected cattle in March 2022. According to the reports, the virus currently isolated in Jordan is also related to the specified Iraqi virus. The spread of the exotic foot-and-mouth disease virus to large susceptible cattle and buffalo populations in Iraq poses risk to numerous animal populations in Iran, Turkey, as well as a number of other countries in the Middle East, and raises serious concern [4].
Close cooperation with the CIS and Middle East countries, trade and economic ties of the Russian Federation with the African countries, where preventive vaccination of livestock against serotype SAT 2 FMDV is not carried out, increase the risk of this serotype FMDV introduction into our country. This is why greater relevance is now placed on the production of the vaccines against serotype SAT 2 FMDV, including SAT2/XIV FMDV, as well as on the development and introduction of diagnostic test systems to assess the effectiveness of the vaccination and the strength of the immunity against this topotype virus in farm and domestic cloven-hoofed animals and to detect post-infection FMDV antibodies in the sera of the unvaccinated animals.
The Federal Centre for Animal Health has developed and successfully tested a mono- and polyvalent vaccine based on the antigen of FMDV strain SAT-2/XIV/2023 [4]. The strain was obtained by adapting the FMDV isolate recovered at the Federal Centre for Animal Health from the bovine pathological material delivered from the Hashemite Kingdom of Jordan in August 2023 to the reproduction in the primarily trypsinized porcine kidney monolayer cell line, in the continuous cell cultures BHK-21/SUSP/ARRIAH, IB-RS-2, PSGK-30, as well as in cattle and pigs. SAT-2/XIV/2023 strain was deposited in the All-Russian State Collection of Exotic FMDV Types and Other Animal Pathogens of the Federal Centre for Animal Health in 2023 and was proposed for the manufacture of vaccine products and diagnostic tools [5][6]. This necessitated the development of an enzyme-linked immunosorbent assay (ELISA) system to objectively and reliably evaluate the antigenic and immunogenic properties of the FMD vaccine based on SAT-2/XIV/2023 virus antigen, as well as the immune background and intensity of post-vaccination immunity in the susceptible animals.
The paper describes the stages of the development and testing of the ELISA system based on an indirect liquid-phase blocking enzyme-linked immunosorbent assay intended for assessment of the humoral immunity against SAT2/XIV FMDV.
MATERIALS AND METHODS
Serum samples. Experimental serum samples collected from cattle, pigs, and white mice served as the ELISA test material.
Preparation of immunospecific reagents. The antigen of FMDV strain SAT-2/XIV/2023 intended for the production of ELISA-specific components (antigen, capture (coating) and detector antibodies) was isolated from the inactivated virus-containing BHK-21 cell suspension according to the following procedure. At the first stage, the antigenic source materials were purified from cellular debris by low-speed centrifugation (Avanti J-26 XP; Beckman Coulter, USA) for 30 minutes. The supernatant was discarded and used to precipitate the viral antigen with 8% polyethylene glycol of molecular weight 6,000 g/mol (PEG 6,000) and 0.85% NaCl for 18–20 hours at (6 ± 2) °C. The precipitated antigen was pelleted using Avanti J-26 XP centrifuge (Beckman Coulter, USA) at 6,000 rpm and 4 °C for 60 minutes. The supernatant was removed, and the pellet was re-suspended in 1/500 of the original volume of the starting material using phosphate buffered saline solution (PBS) at pH 7.4. The resulting suspension was then thoroughly homogenized with 50% chloroform and fractionated at 3,000 rpm and 4 °C for 15 minutes using Allegra X-22R Centrifuge (Beckman Coulter, USA). The upper aqueous fraction was an intermediate substance in the form of a concentrated, partially purified antigen, hereinafter referred to as a precipitate of the antigen of FMDV strain SAT-2/XIV/2023 (Agprecipitate SAT2/XIV). Agprecipitate SAT2/XIV was then fractionated using a discontinuous sucrose density gradient comprising successive layers of 20, 30, 40, 50% sucrose in PBS on Optima L-80 XP Ultracentrifuge (Beckman Coulter, USA) at 24,000 rpm and 4 °C for 3 hours.
The antigenic fractions were analyzed spectrophotometrically at 260 nm wavelength and by electrophoretic separation of protein molecules in the polyacrylamide gel. 30–50% sucrose fractions containing VP1–VP3 SP (an intact FMDV SAT-2/XIV/2023 antigen representing 146S particles), free from impurities or comprising their minimal amount, were combined, labeled as 146S-Ag SAT2/XIV and then used to manufacture the immunospecific components of the liquid-phase blocking ELISA (LPB-ELISA): FMDV capture and detector antibodies as well as antigen.
Rabbits and guinea pigs were immunized twice with 146S-Ag SAT2/XIV at a dose of approximately 0.3 and 0.15 mg/animal, respectively. The antigen intended for administration was mixed with ISA-206 adjuvant in equal proportions. The bleeding was performed on day 35 post the first vaccination.
All animal experiments were conducted in strict compliance with the interstate standard for the accommodation and care of laboratory animals GOST 33215-2014, adopted by the Interstate Council for Standardization, Metrology and Certification, as well as in accordance with the requirements of Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
Standard panel of strain-specific bovine and porcine serum samples was used to select the working dilutions of the LPB-ELISA components, including capture and detector antibodies, antigen and rabbit anti-guinea pig IgG secondary antibody immunoperoxidase conjugate. The panel included both homologous and heterologous samples obtained for other FMDV serotypes, as well as normal serum, collected from the animals not vaccinated against FMD.
ELISA. The test was carried out according to the recommendations of the World Organization for Animal Health (WOAH) for LPB-ELISA [1] and instructions for use of the ELISA test kit for detection of antibodies against FMDV SP using the optimal concentration of immunospecific reagents selected during the test system development. Diagnostic LPB-ELISA test-kit for the detection of antibodies to SAT 2 FMDV (SAT2/VII topotype) SP manufactured by the Federal Centre for Animal Health, as well as a commercial test-kit for the detection of antibodies to serotype SAT 2 FMDV “Solid-phase competitive ELISA for antibodies specific to FMDV serotype SAT 2, hereafter SAT2-IZSLER” (IZSLER & The Pirbright Institute, Italy/UK) were also used according to the manufacturer’s instructions.
The virus neutralization test (VNT) of the FMDV was performed in the continuous porcine kidney cell line IB-RS-2 in accordance with the WOAH recommendations. Microneutralization technique with 96-well flat-bottom cell culture plates was used for the virus neutralization. Approximately 102 TCID50/0.05 mL (tissue culture infective dose of the virus, causing death in 50% of the cells) of the virus was added to the tested and control serum samples two-fold diluted with Eagle’s nutrient medium (permissible range 101.5–102.5 TCID50/0.05 mL. The sera were incubated for 1 hour at 37 °C with 5% CO2, then 0.05 mL of IB-RS-2 cell culture suspensions at a concentration of 10⁶ cells/mL were added to each plate well and incubated under the same conditions for 48–78 hours. The test results were recorded as the virus cytopathic effect (CPE) developed in the control wells with the infected cell culture and without test animal sera. The virus-neutralizing antibody (VNA) titer was defined as the highest serum dilution that inhibits the viral cytopathic effect (CPE) by 50% [1].
Alignment of amino acid sequences. The amino acid sequences of VP1 protein of the FMDV of different SAT 2 topotypes were aligned according to information obtained from GenBank NCBI and the Federal Centre for Animal Health database: SAT2/ETH/2/2022 (GenBank: WKE35517.1); SAT2/JOR/11/2023 (GenBank: WUR05443.1); SAT-2/XIV/2023 (the Federal Centre for Animal Health database); SAT2/ETH/2/91 (GenBank: WKE35516.1); SAT2/ERI/4/98 (GenBank: AAR09103.1); SAT2/LIB/39/2012 (GenBank: AFU55195.1); SAT2/EGY/Ismailia/2018 (GenBank: QZE50286.1); SAT2/EGY/Beni-Suef-2/2018 (GenBank: QEI49588.1); SAT2/EGY/Dakahlia/2017 (GenBank: AXR97922.1) [7][8][9][10][11][12][13][14].
Polyacrylamide gel electrophoresis. Electrophoretic separation of proteins in polyacrylamide gel was performed as previously described [15][16].
Statistical analysis was carried out using online resources, including MedCalc’s Diagnostic test evaluation calculator (https://www.medcalc.org/calc/diagnostic_test.php), Kappa Calculator (https://www.easycalculation.com/statistics/cohens-kappa-index.php).
RESULTS AND DISCUSSION
Development of SAT2/XIV-ARRIAH test system. The effectiveness of the vaccine, immune background and anti-FMDV immunity strength in the susceptible animals are assessed by the level of the specific virus neutralizing (protective) antibodies in the sera of animals after vaccination or infection. In the laboratory diagnostics, two key methods are used to examine serum samples for the antibodies to the FMDV SP: VNT and ELISA [1][2][17][18][19].
The developed ELISA system, designed primarily to evaluate the antigenic and immunogenic activity of the FMD vaccine containing genotype SAT2/XIV FMDV antigen, is based on LPB-ELISA. This ELISA variant, recommended by the WOAH as one of the key methods of retrospective FMD diagnosis aimed at the monitoring the FMD vaccine immunogenicity, assessing the immunity strength, and FMD monitoring tests, has been adapted for the Federal Centre for Animal Health, optimized, and modified. An undeniable advantage of the LPB-ELISA is that the formation of the 'antigen – antibody' complex occurs in the liquid phase, i.e., under conditions that closely mimic the natural environment. In this case, there is no deformation of the virion and the antigenic sites do not change, which makes it similar to the VNT in cell culture or chicken embryonated eggs [1][17][18][19].
When creating the test systems for FMD diagnosis, the developers strive for the ELISA serotype specificity. Indeed, within the serotype, the FMDVs of different genetic lineages, as a rule, reveal a serological relationship. However, when testing the primarily vaccinated young animals, the test systems for detecting the certain serotype FMDV antibodies produced by different manufacturers may demonstrate strain/genotype specificity. This is of great importance in assessing the antigenic and immunogenic activity of a FMD vaccine. It is essential to carefully select the tool to obtain the most objective and reliable information.
Two test systems were earlier developed at the Federal Centre for Animal Health to assess humoral immunity against SAT 2 topotype VII FMDV based on SAT2/LIB/39/2012 and SAT2/ERI/98 strains, which demonstrated a high degree of relatedness and, as a result, interchangeability [3]. These test systems were supposed to be able to provide a full-scale ELISA monitoring of the effectiveness of the vaccine containing the antigen of the new FMDV strain. Data on the genetic and serological matching of SAT 2 FMDV strains and isolates of topotypes VII and XIV were analyzed, and the feasibility of developing a new test system for SAT 2 FMDV of topotype XIV was considered.
To identify the genetic relationship (homology) between topotypes VII and XIV, amino acid sequences of SAT 2 FMDV VP1 protein were compared. As can be seen in Figure 1, the vaccine FMDV strain SAT-2/XIV/2023 had 100% homology with the FMDV isolate recovered in the Hashemite Kingdom of Jordan in 2023 (SAT2/JOR/11/2023) in the amino acid sequence of VP1 polypeptide. Both viruses differed from SAT2/ETH/2/2022 isolate recovered in Ethiopia in 2022 by one amino acid substitution in the G-H loop region and by 20 substitutions from another Ethiopian isolate SAT2/ETH/2/91.
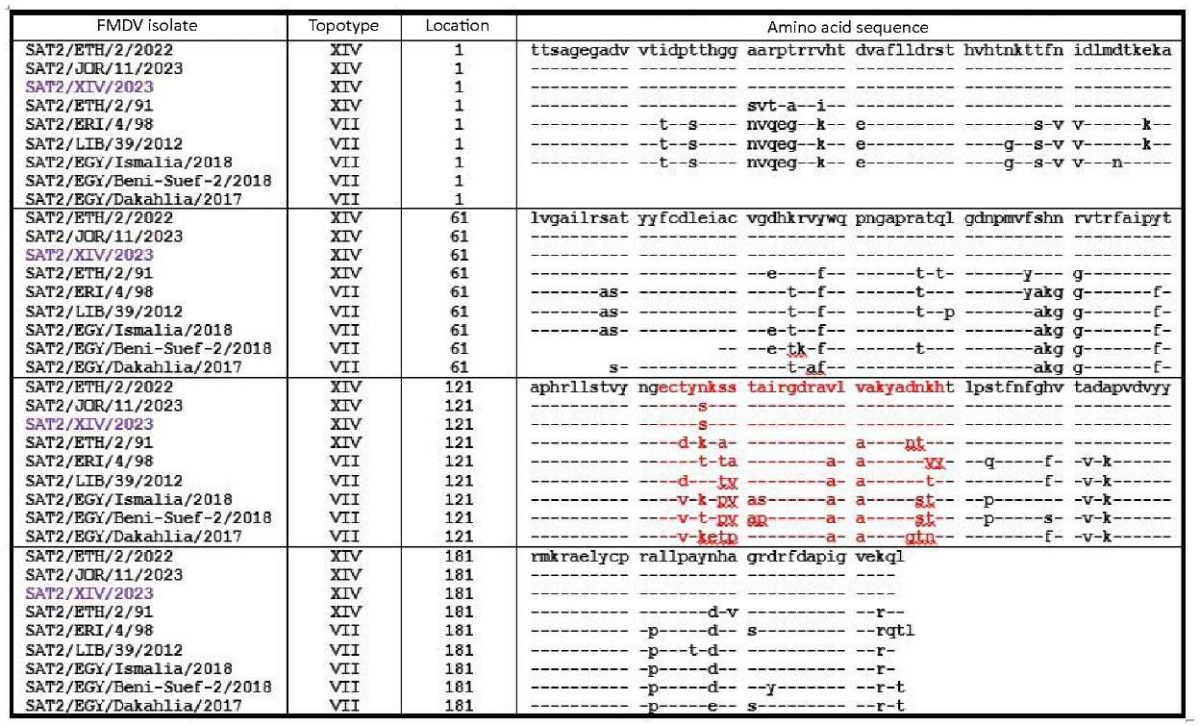
Fig. 1. Amino acid sequences of VP1 polypeptide of serotype SAT 2 topotype XIV and VII FMDV obtained from NCBI and Federal Centre for Animal Health databases (up to 216 a.r.), G-H loop area is highlighted in red
These viruses belong to topotype XIV of SAT 2 serotype and significantly differ from the isolates of SAT 2 topotype VII virus, which directly affects the antigenic matching of SAT 2 virus strains belonging to different topotypes. The phylogenetic tree of the SAT 2 FMDV in Figure 2, based on the comparison of VP1 gene nucleotide sequences clearly demonstrates the topotypic (genotypic) differences between the virus strains.
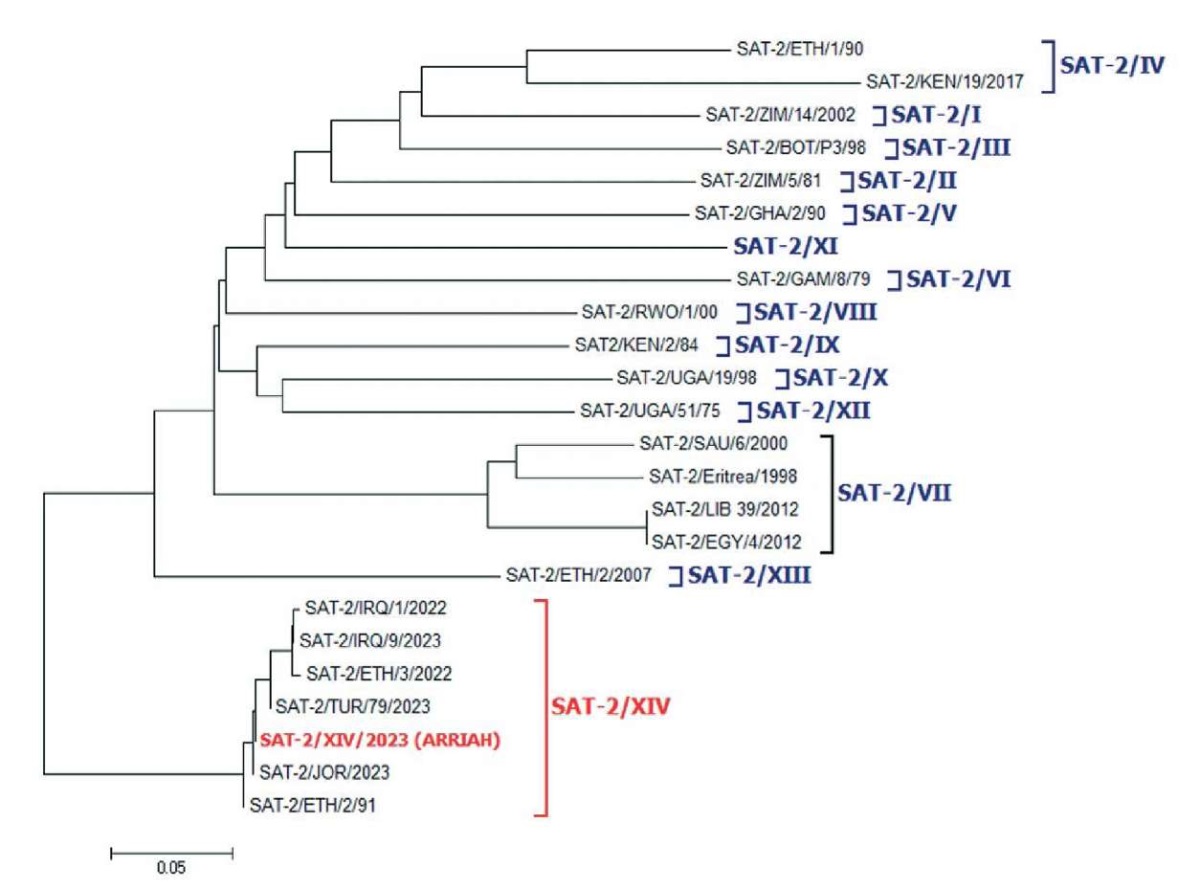
Fig. 2. Location of SAT-2/XIV/2023 strain on serotype SAT 2 FMDV phylogenetic tree. The dendrogram is based on the comparison of VP1 gene nucleotide sequences. Topotypes I–XIV are in square brackets
When studying the antigenic matching of the vaccine strain SAT-2/XIV/2023 (topotype XIV) and FMDV strains SAT2/LIB/39/2012 (topotype VII) and SAT2/ERI/98 (topotype VII), a low degree of relatedness between the strains of different topotypes was established in VNT in IB-RS-2 cell culture: 0.06–0.16. Meanwhile, strains SAT2/LIB/39/2012 and SAT2/ERI/98 showed close relatedness between each other: 0.36 and 0.69, respectively (Table 1), which indicated good cross-protection between these FMDVs.
Table 1
Antigenic relatedness of subtype SAT 2 FMDV strains determined in the virus neutralization test, r1
|
Serum samples |
r1mean in FMDV neutralization test* |
||
|
SAT2/LIB/39/2012 |
SAT2/ERI/98 |
SAT-2/XIV/2023 |
|
|
SAT2/LIB/39/2012 |
1.0 |
0.36 |
0.06 |
|
SAT2/ERI/98 |
0.69 |
1.0 |
0.16 |
|
SAT-2/XIV/2023 |
0.14 |
0.16 |
1.0 |
|
* r1 ≥ 0.3 – close relatedness; r1 < 0.3 – low relatedness. |
|||
Thus, when developing an enzyme-linked immunosorbent assay system to assess the antigenicity and immunogenicity of the vaccine against SAT2/XIV FMD, the production strain SAT-2/XIV/2023 was selected.
Special attention was paid to the extraction of 146S component from the inactivated virus-containing suspension of BHK-21 cell culture (146S-Ag SAT2/XIV) when preparing specific LPB-ELISA reagents for the detection of antibodies to SAT2/XIV FMDV SP. It is an intact antigen with sedimentation coefficient of 146S, that is, an antigen with an unaltered structure, representing virions that have lost their infectivity during the inactivation process. This antigen consists of a capsid enclosing inactivated RNA. The integrity of the capsid ensures the immunogenicity of the FMD vaccine, since the surface polypeptides VP1–VP3 induce the production of the virus neutralizing strain/serotype-specific antibodies. Use of highly purified 146S antigen during the ELISA "liquid phase", when an immune complex with specific antibodies is formed in the test sample, as well as for the immunization of rabbits and guinea pigs for the production of capture (coating) and detector antibodies, respectively, allows for the determination of the level of humoral immunity against FMD with the highest possible reliability.
The use of antigens with a lower degree of purification in the ELISA, i.e. partially purified antigen (antigenic precipitate) or antigen-containing cell suspension, which are characterized by the presence of ballast proteins in the form of residual cell debris and serum albumin, can lead to biased test results.
During antigen fractionation by ultracentrifugation in a 20–50% sucrose gradient, the majority of 146S particles accumulated at the interface between the 30 and 40% sucrose layers as an opalescent band. The gradient fractions collected at 1 mL were analyzed spectrophotometrically at a wavelength of 260 nm to construct SAT2/XIV Agprecipitate sedimentation profile and by electrophoretic separation of protein molecules in a 12% poly-acrylamide gel (Fig. 3B).
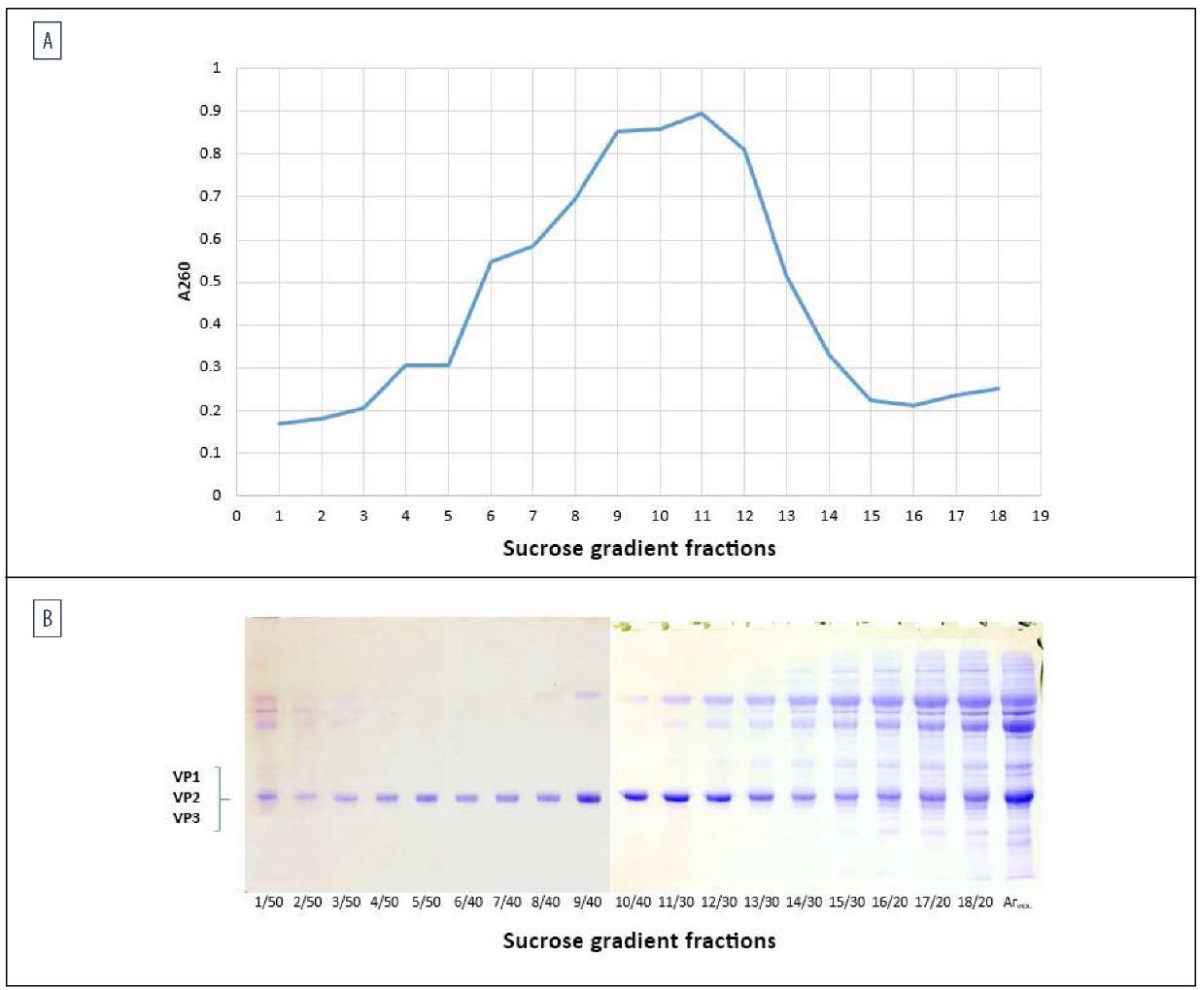
Fig. 3. Isolation of 146S-Ag SAT2/XIV during fractionation of SAT2/XIV Agprecipitate using sucrose gradient ultracentrifugation:
A – sedimentation profile of SAT2/XIV Agprecipitate with wavelength 260 nm at 1:10 dilution;
B – electrophoregram of sucrose gradient fractions, including SP VP1, VP2, VP3 of SAT-2/XIV/2023
Fractions with the highest accumulation of VP1–VP3 structural polypeptides and the lowest content or absence of impurities were selected for 146S antigen. As a result, after combining the fractions, 146S-Ag SAT2/XIV was obtained with a protein concentration of approximately 0.55 mg/mL. Figure 4 shows an electrophoregram of SAT-2/XIV/2023 FMDV antigen in 12% polyacrylamide gel before and after fractionation by sucrose-gradient ultracentrifugation.
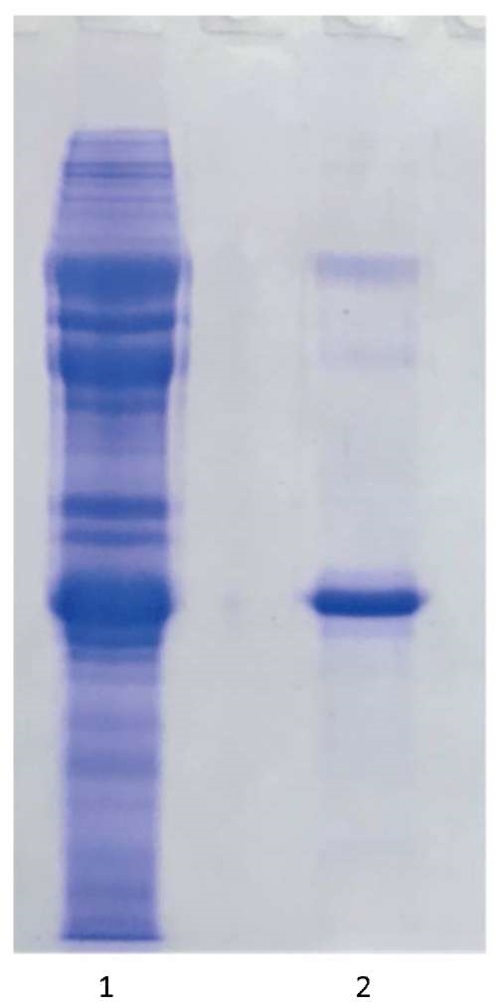
Fig. 4. 12% polyacrylamide gel electrophoresis of SAT-2/XIV/2023 FMDV antigen:
1 – SAT2/XIV Agprecipitate before fractionation by sucrose gradient ultracentrifugation;
2 – 146S-Ag SAT2/XIV
The resulting 146S-Ag SAT2/XIV was used to manufacture immunospecific components of LPB-ELISA: capture and detector antibodies, and FMDV antigen.
The test system for the determination of SAT2/XIV FMDV SP antibodies demonstrated specificity and sensitivity in determining the immune status of the tested animals when using immunospecific reagents in the following working dilutions: capture antibodies – 1:1000, detector antibodies – 1:5000, FMDV antigen – 1:1000, or 0.55 µg/mL, commercial anti-species conjugate – 1:1500, or 0.75 µg/mL.
When interpreting the ELISA results, it is necessary to establish qualitative assessment criteria. For this purpose, the cut-off value is determined – a positive and negative threshold (PNT) that allows classifying the tested biological samples as positive or negative for a specific infectious agent.
In the developed SAT2/XIV-ARRIAH test system, the qualitative analysis of the ELISA results is based on a PNT of 50% PI (percentage of inhibition) [1]. The calculation was performed based on the data obtained during testing of 176 serum samples collected from clinically healthy cattle and pigs not vaccinated against FMD. Mean PI and standard deviation from the mean (SD) were obtained. The mean PI for this serum sample panel (n = 176) was 21.09%, SD – 14.67%. The PNT was calculated by the formula: PNT = PImean + 2SD and amounted to 50.43%.
Thus, the PNT value for the qualitative characteristics of the serum samples tested for the presence of SAT2/XIV FMDV antibodies using the proposed test system corresponded to the predicted 50% PI, which indicated the optimal choice of working dilutions of the immunospecific components that determined the accuracy and objectivity of the analysis.
Trials of SAT2/XIV-ARRIAH test system. SAT2/XIV-ARRIAH ELISA system was evaluated during the assessment of the antigenicity and immunogenicity of SAT2/XIV monovalent adsorbed FMD vaccine in comparison with other test systems for SAT 2 FMD diagnosis (Table 2). For this purpose, fifteen black-and-white bull calves were vaccinated once with the vaccine at different doses: whole dose, 1/4 dose and 1/16 dose, with five animals per each vaccine dose. One control animal was not vaccinated. Blood for testing in ELISA and VNT was collected from all animals on day 0, 3, 7, 14, 21 post administration of the vaccine against SAT2/XIV FMD. The serum samples were tested for SAT 2 FMDV antibodies using the following ELISA systems: SAT2/XIV-ARRIAH based on SAT-2/XIV/2023 strain; SAT2/VII-ARRIAH based on SAT2/LIB/39/2012 strain; SAT2-IZSLER, and SAT-2/XIV/2023 VNT.
Table 2
ELISA and VNT results of serum samples collected from cattle vaccinated against SAT2/XIV FMD (adsorbed monovalent vaccine)
|
Serum samples |
Vaccine dose |
Test system |
|||
|
SAT2/XIV-ARRIAH PIpos ≥ 50%, pos/n |
SAT2/VII-ARRIAH PIpos ≥ 50%, pos/n |
SAT2-IZSLER PIpos ≥ 70%, pos/n |
SAT2/XIV-VNT Тpos VNA ≥ 5 log2, pos/n |
||
|
Sbov 0 dpv |
before vaccination |
0/16 |
0/16 |
0/16 |
0/16 |
|
Sbov 3 dpv |
whole dose |
0/5 |
0/5 |
0/5 |
0/5 |
|
1/4 |
0/5 |
0/5 |
0/5 |
0/5 |
|
|
1/16 |
0/5 |
0/5 |
0/5 |
0/5 |
|
|
control |
0/1 |
0/1 |
0/1 |
0/1 |
|
|
Sbov 7 dpv |
whole dose |
1/5 |
0/5 |
0/5 |
0/5 |
|
1/4 |
1/5 |
0/5 |
0/5 |
0/5 |
|
|
1/16 |
1/5 |
1/5 |
0/5 |
0/5 |
|
|
control |
0/1 |
0/1 |
0/1 |
0/1 |
|
|
Sbov 14 dpv |
whole dose |
5/5 |
4/5 |
3/5 |
3/5 |
|
1/4 |
4/5 |
2/5 |
1/5 |
3/5 |
|
|
1/16 |
2/5 |
1/5 |
0/5 |
1/5 |
|
|
control |
0/1 |
0/1 |
0/1 |
0/1 |
|
|
Sbov 21 dpv |
whole dose |
5/5 |
4/5 |
3/5 |
4/5 |
|
1/4 |
4/5 |
1/5 |
1/5 |
2/5 |
|
|
1/16 |
2/5 |
1/5 |
0/5 |
1/5 |
|
|
control |
0/1 |
0/1 |
0/1 |
0/1 |
|
|
Sbov – bovine sera; PI – inhibition percent; Тpos VNA – positive (protective) titre of virus neutralizing antibodies; pos/n – number of positive reactors against total number of the animals in the group; dpv – day post vaccination. |
|||||
As can be seen from Table 2, the effectiveness of SAT2/XIV-ARRIAH test system was higher in recording the specific antibody production as compared to other test systems under study. Already on day 7, one seropositive animal was detected in each of the three groups of vaccinated cattle, and one positive reactor was detected using SAT2/VII-ARRIAH test system. In the other two reactions, the specific antibodies were detected only on day 14. As a result, the number of positive reactors detected using ELISA and VNT on day 14–21 post administration of different vaccine doses was the following: SAT2/XIV-ARRIAH – 22/30 (73.3%), SAT2/VII-ARRIAH – 13/30 (43.3%), SAT2-IZSLER – 8/30 (26.7%), SAT2/XIV-VNT – 14/30 (46.7%), which indicated an undeniable advantage of SAT2/XIV-ARRIAH test system as for its diagnostic sensitivity.
An experimental saponin-containing vaccine with inactivated FMDV strain SAT-2/XIV/2023 also induced humoral response when administered to the laboratory white mice. Animals weighing 24–26 g were vaccinated once with the vaccine at different doses: whole dose, 1/4 dose and 1/16 dose, at 0.4 mL per animal (13 animals for each vaccine dose). Eleven control mice were not vaccinated. Blood was collected in pools on days 14 and 21 after vaccination. The results are presented in Table 3. As in the experiment with cattle, SAT2/XIV-ARRIAH test system demonstrated higher sensitivity as compared to other test systems for SAT2/VII topotype.
Table 3
ELISA results of serum samples collected from white mice immunized with an adsorbed monovalent vaccine against SAT2/XIV FMDV
|
Serum samples |
Vaccine dose |
Test system |
||
|
SAT2/XIV-ARRIAH PIpos ≥ 50% |
SAT2/VII-ARRIAH PIpos ≥ 50% |
SAT2-IZSLER PIpos ≥ 70% |
||
|
Smur 14 dpv |
whole dose |
67.5% |
64.4% |
30.6% |
|
1/4 |
64.9% |
50.1% |
14.9% |
|
|
1/16 |
21.1% |
5.9% |
22.3% |
|
|
Smur 21 dpv |
whole dose |
71.6% |
65.3% |
32.5% |
|
1/4 |
63.5% |
48.0% |
43.7% |
|
|
1/16 |
12.5% |
1.7% |
1.8% |
|
|
control |
6.1% |
0.35% |
2.4% |
|
|
Smur – white mouse sera; PI – inhibition percent; dpv – day post vaccination; positive PI values are in bold. |
||||
The vaccine containing SAT-2/XIV/2023 FMDV antigen, as well as SAT2/XIV-ARRIAH diagnostic test kit supplied with the vaccine, were tested in the field, namely in Jordan. According to the data provided by the Jordanian side, both the monovalent adsorbed vaccine against SAT2/XIV and the homologous test system for evaluating the effectiveness of manufactured by the Federal Centre for Animal Health vaccine demonstrated convincing results of the vaccine’s antigenicity after double vaccination of cattle. All vaccinated animals (10 animals) demonstrated protective antibody level with PImean = 84.58 ± 12.56, while the unvaccinated controls (2 animals) did not show any antibodies to the SAT2/XIV FMDV (PImean = 35.34 ± 2.21).
To confirm the reliability of the ELISA data, 163 serum samples collected from pigs and cattle were tested using the following test systems: SAT2/XIV-ARRIAH, SAT2/VII-ARRIAH, SAT2-IZSLER. The results of parallel serum tests before and after whole-dose vaccination against SAT2/XIV FMD agent (vaccine based on strain SAT-2/XIV/2023) and SAT2/VII FMD agent (vaccines based on strains SAT2/LIB/39/2012 and SAT2/ERI/98), as well as after challenge with FMDV strain SAT2/ERI/98 are shown in Table 4.
Table 4
ELISA results of serum samples collected from cattle and pigs before and after vaccination against serotype SAT 2 FMD and challenge
|
Samples serum |
Test system |
||||||||
|
SAT2/XIV-ARRIAH PIpos ≥ 50% |
SAT2/VII-ARRIAH PIpos ≥ 50% |
SAT2-IZSLER PIpos ≥ 70% |
|||||||
|
PImean |
pos/n |
SP, % |
PImean |
pos/n |
SP, % |
PImean |
pos/n |
SP, % |
|
|
Spor 0 dpv |
23.2 |
0/16 |
– |
13.9 |
0/16 |
– |
22.3 |
0/16 |
– |
|
Sbov 0 dpv |
25.1 |
0/37 |
– |
30.3 |
0/37 |
– |
20.2 |
0/37 |
– |
|
Spor 16–28 dpv SAT2/LIB/39/2012 |
68.2 |
28/30 |
93.3 |
83.7 |
29/30 |
96.7 |
90.2 |
29/30 |
96.7 |
|
Sbov 14–35 dpv SAT2/LIB/39/2012 |
70.1 |
14/15 |
93.3 |
89.5 |
15/15 |
100.0 |
87.6 |
15/15 |
100.0 |
|
Sbov 21–45 dpv/dpi SAT2/ERI/98 |
59.5 |
23/35 |
65.7 |
80.3 |
27/35 |
77.1 |
89.1 |
29/35 |
82.9 |
|
Sbov 14–21 dpv SAT-2/XIV/2023 |
76.8 |
30/30 |
100.0 |
63.8 |
24/30 |
80.0 |
72.6 |
18/30 |
60.0 |
|
Sbov – bovine sera; Spor – porcine sera; PI – inhibition percent; pos/n – number of positive reactors against total number of the animals in the group; SP – seropositivity (number of positive reactors); dpv/dpi – day post vaccination/infection. |
|||||||||
The analysis of the resulted data demonstrated pronounced topotype specificity of the test systems for this panel of samples. The bovine sera against FMDV SAT-2/XIV/2023 antigen tested positive in 100% of cases using ELISA SAT2/XIV-ARRIAH test system, and in 80 and 60% cases using SAT2/VII-ARRIAH and SAT2-IZSLER, respectively. While seropositivity of animals against SAT2 topotype VII FMDV in SAT2/VII-ARRIAH and SAT2-IZSLER test systems was 91.27 and 93.2%, respectively, in SAT2/XIV-ARRIAH test system it amounted to 84.1%.
Determining diagnostic parameters of SAT2/XIV-ARRIAH test system. During SAT2/XIV-ARRIAH validation, such basic diagnostic parameters as sensitivity, specificity, accuracy, and the κ-criterion were determined as described earlier [20]. Table 5 shows the data on statistical processing of ELISA results for 301 serum samples collected from unvaccinated cattle and from cattle vaccinated against SAT2/XIV FMDV.
Table 5
Diagnostic parameters of the test systems for detecting antibodies to SAT 2 FMDV SP, defined for the SAT2/XIV topotype
|
Test systems used |
Diagnostic parameters (n = 301), (95% confidence interval) |
|||
|
sensitivity |
specificity |
accuracy |
κ-criterion* |
|
|
SAT2/XIV-ARRIAH |
90.24% (83.58–94.86%) |
98.31% (95.15–99.65%) |
95.02% (91.91–97.18%) |
0.896 |
|
SAT2/VII-ARRIAH |
55,0% (41.6–67.9%) |
98,1% (89.9–100.0%) |
75.2% (66.2–82.9%) |
0.516 |
|
SAT2-IZSLER |
40,0% (27.6–53.5%) |
100,0% (93.3–100.0%) |
68.1% (58.7–76.6%) |
0.385 |
|
*κ-criterion – consistency between ELISA test results of individual serum samples and the animals' diagnostic status; < 0 – no consistency; 0–0.20 – insignificant; 0.21–0.40 – low; 0.41–0.60 – moderate; 0.61–0.80 – significant; 0.81–1.00 – high. |
||||
As evident from the results, 90% diagnostic sensitivity, 98% diagnostic specificity, and 95% diagnostic accuracy of SAT2/XIV-ARRIAH ELISA system demonstrated a high degree of consistency between the ELISA results and the known diagnostic status of the tested animals (κ-criterion – 0.896).
Currently only one test system for retrospective FMD diagnosis, capable of detecting SAT2 FMDV SP antibodies using ELISA is reliably known to be available on the global market. This is Solid-phase competitive ELISA test kit for antibodies specific to FMDV serotype SAT 2 produced by IZSLER & The Pirbright Institute (Italy/United Kingdom) [21].
The use of monoclonal antibodies in diagnostic ELISA systems for serotyping specific FMDV antibodies is designed to create the versatility of analysis within a specific FMDV serotype (the possibility of detecting antibodies induced against different FMDV strains of the same serotype in the tested sera or blood plasma with the same sensitivity), that is, the test system should have wide serotype specificity.
However, in the case of SAT2-IZSLER test system, pronounced topotype specificity was observed. When testing samples of monospecific bovine or porcine sera against SAT2/VII and SAT2/XIV FMDV using SAT2-IZSLER, the highest number of positive results was obtained for sera against topotype SAT2/VII, while antibodies to topotype SAT2/XIV were detected with lower efficiency than in case of SAT2/XIV-ARRIAH test system. This indicates that the use of SAT2-IZSLER kit to study the antigenicity and immunogenicity of the FMD vaccine comprising SAT-2/XIV/2023 FMDV antigen, does not allow for an objective and reliable assessment of the vaccine effectiveness using ELISA. Thus, the ELISA system for assessing humoral immunity against SAT2/XIV FMDV, which has a 100% degree of homology with the vaccine strain and high diagnostic performance, is an indispensable and currently irreplaceable tool for evaluating vaccine quality in ELISA.
CONCLUSION
The developed test system based on a liquid-phase blocking ELISA variant for detection of SAT2/XIV FMDV SP antibodies is specific and sensitive. The uniqueness of the test system consists in a 100% degree of homology with the vaccine products, which allows for the most reliable and effective assessment of the antigenic and immunogenic properties of the vaccine during its production and subsequent use in the field. During validation trials, this test system demonstrated high diagnostic sensitivity (90%), diagnostic specificity (98%), and diagnostic accuracy (95%), indicating a strong consistency between ELISA results and the known diagnostic status of the tested animals (κ-criterion – 0.896).
Contribution of the authors: Lugovskaya N. N. – development and validation of test systems, serum sample ELISA testing, statistical analysis of results, scientific literature review, research conceptualization, and manuscript preparation; El’kina Yu. S. – experiment design and implementation in cattle, collection of blood samples for ELISA and virus neutralization test; Shevchenko M. A. – experiment design and implementation in cattle, collection of blood samples for ELISA and virus neutralization test; Gochmuradov Y. M. – experiment design and implementation in cattle, collection of blood samples for ELISA and virus neutralization test; Klyukina N. D. – experiment design and implementation in mice, collection of blood samples for ELISA and virus neutralization test; Mikhalishin D. V. – overall leadership, consulting in vaccine prevention, material resource provision; Borisov A. V. – overall leadership, material resource provision.
Вклад авторов: Луговская Н. Н. – разработка и валидация тест-систем, исследования образцов сыворотки крови в ИФА, статистический анализ результатов, изучение научных публикаций по теме, разработка концепции исследования и подготовка статьи; Елькина Ю. С. – организация и проведение опыта на КРС, отбор крови для исследований в ИФА и РН; Шевченко М. А. – организация и проведение опыта на КРС, отбор крови для исследований в ИФА и РН; Гочмурадов Ы. М. – организация и проведение опыта на КРС, отбор крови для исследований в ИФА и РН; Клюкина Н. Д. – организация и проведение опыта на лабораторных мышах, отбор крови для исследований в ИФА и РН; Михалишин Д. В. – общее руководство, консультирование по вопросам вакцинопрофилактики, обеспечение материальной базы; Борисов А. В. – общее руководство, обеспечение материальной базы.
References
1. WOAH. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 13th ed. 2024. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm
2. Ferrari G., Paton D., Duffy S., Bartels C., Knight-Jones T. Foot and mouth disease vaccination and post-vaccination monitoring: Guidelines. Ed. by S. Metwally, S. Münstermann. Rome: FAO, OIE; 2016. 111 p. https://openknowledge.fao.org/handle/20.500.14283/i5975e
3. Lugovskaya N. N., Silantyeva E. A., Mikhalishin D. V., Moroz N. V., Malygin M. P., Kharitonova A. A., et al. Diagnostic test-system for detection of antibodies to structural proteins of FMD virus genotype SAT2/VII in the liquid-phase blocking ELISA. Actual Questions of Veterinary Biology. 2023; (4): 43–52. https://doi.org/10.24412/2074-5036-2023-4-43-52 (in Russ.)
4. ProMED, International society for infectious diseases. https://promedmail.org/promed-post/?id=20230204.8708168
5. Mikhalishin D. V., Doronin M. I., Chvala I. A., Borisov A. V., Kharitonova A. A., Gochmuradov Y. М., et al. Culture inactivated emulsion vaccine against foot- and-mouth disease of SAT2/XIV genotype from SAT-2/XIV/2023 strain. Patent No. 2824660 Russian Federation, Int. Cl. A61K 39/135 (2006.01). Federal Centre for Animal Health. No. 2024108919. Date of filing: 03.04.2024. Date of publication: 12.08.2024. Bull. No. 23. (in Russ.)
6. Nikiforov V. V., Doronin M. I., Borisov A. V., Chvala I. A., Mikhalishin D. V., Fomina S. N., et al. SAT-2/XIV/2023 strain of foot and mouth disease virus Aphtae epizooticae of SAT2/XIV genotype for production of biopreparations for diagnosis and specific prevention of foot-and-mouth disease. Patent No. 2817257 Russian Federation, Int. Cl. C12N 7/00 (2006.01). Federal Centre for Animal Health. No. 2023123055. Date of filing: 04.09.2023. Date of publication: 12.04.2024. Bull. No. 11. (in Russ.)
7. Knowles N. J., Wadsworth J., Hicks H. M., Polo N., Mioulet V., Gizaw D., King D. P. Genome sequences of SAT 2 foot-and-mouth disease viruses belonging to topotype XIV. Submitted (28-FEB-2023) Vesicular Disease Reference Laboratory Group, The Pirbright Institute, United Kingdom. https://www.ncbi.nlm.nih.gov/protein/WKE35516.1
8. Knowles N. J., Wadsworth J., Hicks H. M., Polo N., Mioulet V., Gizaw D., King D. P. Genome sequences of SAT 2 foot-and-mouth disease viruses belonging to topotype XIV. Submitted (28-FEB-2023) Vesicular Disease Reference Laboratory Group, The Pirbright Institute, United Kingdom. https://www.ncbi.nlm.nih.gov/protein/WKE35517.1
9. Abualghusein I. H. M., Ababneh M. M. K., Al-Zghoul M. B., Alghizzawi D. A. A., Aboomer H. A. A. Submitted (07-JAN-2024) Basic Veterinary Science, Jordan University of Science and Technology, Jordan. https://www.ncbi.nlm.nih.gov/protein/WUR05443.1
10. Sahle M., Venter E. H., Dwarka R. M., Vosloo W. Genetic heterogeneity of SAT-2 type foot-and-mouth disease viruses in East Africa. Submitted (17-JUL-2003) Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria, South Africa. https://www.ncbi.nlm.nih.gov/protein/AAR09103
11. Ahmed H. A., Salem S. A. H., Habashi A. R., Arafa A. A., Aggour M. G. A., Salem G. H., et al. Emergence of foot-and-mouth disease virus SAT 2 in Egypt during 2012. Transboundary and Emerging Diseases. 2012; 59 (6): 476–481. https://doi.org/10.1111/tbed.12015
12. Hassan A. M., El-Mayet F. S., El-Habbaa A. S., Shahein M. A., El Zowalaty M. E., Hagag N. M., Sharawi S. S. A. Molecular characterization of newly emerging foot- and-mouth disease virus serotype SAT 2 of Lib-12 lineage isolated from Egypt. Virus Research. 2022; 311: 198651. https://doi.org/10.1016/j.virusres.2021.198651
13. Hosein H. I., Rouby S. R., Hendy K. Submitted (23-MAY-2019) Veterinary Medicine, Faculty of Veterinary Medicine, Egypt. https://www.ncbi.nlm.nih.gov/protein/QEI49588.1
14. Abdulrahman D. A., Shaheen M. A., El-Deeb A. H., Shafik N. G., Hussein H. A. Characterization of FMDVs from vaccinated cattle and buffaloes in Egypt 2015–2017. Submitted (20-NOV-2017) Virology, Animal Health Research Institute (AHRI), Egypt. https://www.ncbi.nlm.nih.gov/protein/AXR97922.1
15. Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227: 680–685. https://doi.org/10.1038/227680a0
16. Lugovskaya N. N., Scherbakov A. V., Yakovleva A. S., Tsyvanyuk M. A., Mudrak N. S., Drygin V. V., Borisov A. V. Detection of antibodies to avian infectious virus by a recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. Journal of Virological Methods. 2006; 135 (2): 292–296. https://doi.org/10.1016/j.jviromet.2006.03.019
17. Hamblin C., Barnett I. T. R., Hedger R. S. A new enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against foot-and-mouth disease virus: I. Development and method of ELISA. Journal of Immunological Methods. 1986; 93 (1): 115–121. https://doi.org/10.1016/0022-1759(86)90441-2
18. Hamblin C., Barnett I. T. R., Crowther J. R. A new enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against foot-and-mouth disease virus: II. Application. Journal of Immunological Methods. 1986; 93 (1): 123–129. https://doi.org/10.1016/0022-1759(86)90442-4
19. Hamblin C., Kitching R. P., Donaldson A. I., Crowther J. R., Barnett I. T. R. Enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against foot-and-mouth disease virus: III. Evaluation of antibodies after infection and vaccination. Epidemiology and Infection. 1987; 99 (3): 733–744. https://doi.org/10.1017/S0950268800066590
20. Lugovskaya N. N., Kalinina Ye. N., Malkova K. S., Vorobyova O. V., Goryacheva G. M., Mayorova T. K. Validation of the technique aimed at the determination of FMD antibodies in liquid phase blocking sandwich ELISA. Veterinary Science Today. 2015; (3): 22–29. https://elibrary.ru/umsjvj (in Russ.)
21. Dho G., Grazioli S., Bugnetti M., Pezzoni G., Maree F. F., Esterhuysen J., et al. Ready-to-use kits for detection of antibody to FMDV serotypes SAT1 and SAT2. Open session of the Standing Technical and Research Committees of the EuFMD (Cavtat, Croatia; October 29–31, 2014). https://www.fao.org/fileadmin/user_upload/eufmd/Open_Session_2014PPTS/Parallel29oct/WPar8.pdf
About the Authors
N. N. LugovskayaRussian Federation
Natalia N. Lugovskaya - Cand. Sci. (Biology), Leading Researcher, Laboratory for FMD Prevention, Federal Centre for Animal Health.
6 Gvardeyskaya str., Yur’evets, Vladimir 60090
Yu. S. El’kina
Russian Federation
Yulia S. El’kina - Cand. Sci. (Veterinary Medicine), Junior Researcher, Laboratory for FMD Prevention, Federal Centre for Animal Health.
6 Gvardeyskaya str., Yur’evets, Vladimir 60090
M. A. Shevchenko
Russian Federation
Maxim A. Shevchenko - Cand. Sci. (Veterinary Medicine), Junior Researcher, Laboratory for FMD Prevention, Federal Centre for Animal Health.
6 Gvardeyskaya str., Yur’evets, Vladimir 60090
Y. M. Gochmuradov
Russian Federation
Ykhlas M. Gochmuradov - Veterinarian, Laboratory for FMD Prevention, Federal Centre for Animal Health.
6 Gvardeyskaya str., Yur’evets, Vladimir 60090
N. D. Klyukina
Russian Federation
Nadezhda D. Klyukina - Cand. Sci. (Veterinary Medicine), Senior Researcher, Laboratory for FMD Prevention, Federal Centre for Animal Health.
6 Gvardeyskaya str., Yur’evets, Vladimir 60090
D. V. Mikhalishin
Russian Federation
Dmitry V. Mikhalishin - Dr. Sci. (Veterinary Medicine), Chief expert in FMD vaccine prevention, Federal Centre for Animal Health.
6 Gvardeyskaya str., Yur’evets, Vladimir 60090
A. V. Borisov
Russian Federation
Alexey V. Borisov - Cand. Sci. (Veterinary Medicine), Head of Laboratory for FMD Prevention, Federal Centre for Animal Health.
6 Gvardeyskaya str., Yur’evets, Vladimir 60090
Review
For citations:
Lugovskaya N.N., El’kina Yu.S., Shevchenko M.A., Gochmuradov Y.M., Klyukina N.D., Mikhalishin D.V., Borisov A.V. Development and application of ELISA test system for assessing humoral immunity against SAT2 topotype XIV foot-and-mouth disease virus. Veterinary Science Today. 2025;14(3):283-293. https://doi.org/10.29326/2304-196X-2025-14-3-283-293



































