Scroll to:
Rabies in the Russian Federation: A 35-year review of trends, patterns, and influencing factors
https://doi.org/10.29326/2304-196X-2025-14-3-232-240
Abstract
Introduction. Sylvatic rabies cases characterized by a consistently high incidence among wild and domestic animals are reported in the Russian Federation. The epizootic cycle of rabies is maintained through the biological reservoir of Lyssavirus rabies in wild canid predators, primarily the red fox (Vulpes vulpes). Fox ecology and behavior determine the spatial spread of rabies, its seasonal incidence patterns, and the species composition of animals involved in the epizootic cycle. Objective. The rabies spatiotemporal analysis of 35-year monitoring data to study determinants and patters of the current disease situation.
Materials and methods. Using Microsoft Access (relational database management system www.microsoft.com) the data on rabies outbreaks in the Russian Federation, rabies vaccination among wild animals and natural and agricultural zoning were aggregated. For spatial analysis, all epizootiological data were geocoded and visualized as vector map layers within the GIS thematic project. The GIS project was constructed using QGIS Desktop platform (www.qgis.org).
Results. The current rabies distribution area covers most of the Russian regions. The area of persistently high rabies incidence primarily encompasses the forest-steppe, mixed forest, and broadleaf forest biomes of the East European Plain. In the Russian Federation, the maximum number of rabies cases is reported among foxes. Rabies epizootics in natural ecosystems exhibit spillover effects, leading to active transmission among multiple domestic animal species. The primary risk group involves dogs, cats and cattle. Oral rabies vaccination of wild carnivores established a significant downward trend in animal rabies incidence while reducing amplitude fluctuations in long-term epizootic cycle.
Conclusion. The observed decline in rabies incidence across the Russian Federation has not been accompanied by a proportional reduction in the disease’s geographic distribution. These findings underscore the need to modify current control measures and implement a comprehensive program for complete elimination of circulating Lyssavirus rabies strains from infected ecosystems.
Keywords
For citations:
Gulyukin A.M., Shabeykin A.A. Rabies in the Russian Federation: A 35-year review of trends, patterns, and influencing factors. Veterinary Science Today. 2025;14(3):232-240. https://doi.org/10.29326/2304-196X-2025-14-3-232-240
INTRODUCTION
Rabies is a viral disease of the sylvatic cycle that causes fatal meningoencephalitis in all mammalian species [1]. Clinical rabies in animals and humans can be induced by infection with any of the 18 known types of viruses of Lyssavirus genus [2]. Lyssaviruses circulate within conspecific populations of mammals, known as biological reservoirs, and are maintained in natural ecosystems. The vast majority of lyssaviruses circulate in bat populations (Chiroptera) [3]. An exception is the Lyssavirus rabies species, which possessed biological mechanisms that ensure the replacement of the reservoir host [4][5][6]. The adaptation of Lyssavirus rabies to sustained circulation within terrestrial Carnivora populations has facilitated its global spread and establishment in diverse ecosystems worldwide [7].
Lyssavirus rabies is responsible for the overwhelming majority of all reported rabies cases [8][9]. Since the mid-20th century, the red fox (Vulpes vulpes) has served as the primary reservoir host and main vector for Lyssavirus rabies transmission [10][11]. In Arctic ecosystems, the Arctic fox (Alopex lagopus) is the primary reservoir for Lyssavirus rabies [12]. In forest biomes, the Lyssavirus rabies may be maintained in fox and raccoon dog (Nyctereutes procyonoides) populations [13]. However, the role of raccoon dogs as a key biological reservoir of rabies virus in the Russian Federation remains debated [14].
Although sylvatic (fox) rabies results in fewer human infections compared to urban canine rabies, it causes significant morbidity and mortality among diverse domestic and wild animal species [15]. Infection of non-reservoir animal species represents a spillover event that terminates the epizootic chain, as these dead-end hosts cannot sustain further transmission [16].
It was proved that three species of lyssaviruses are circulating in bat populations in the Russian Federation [17][18][19]. However, given the diversity of bat species and their habitats, this list is likely incomplete. Strains of European bat lyssavirus 1 (EBLV-1, formerly Lyssavirus hamburg) have been confirmed to circulate in serotine bat (Eptesicus serotinus) populations across the European subcontinent. The circulation of Lyssavirus Irkut virus strains was detected in a population of the greater tube-nosed bat (Murina leucogaster). In the Russian Federation, the distribution of this bat species and its maintained lyssavirus extends into climatically suitable areas of Siberia and the Far East. In the foothills of the Caucasus, the circulation of Lyssavirus caucasicus strains (West Caucasian bat lyssavirus) was detected in the population of the common bent-wing bat (Miniopterus schreibersii).
Due to the largely isolated nature of insectivorous bat colonies, lyssavirus transmission from bats to other species or humans occurs only sporadically and does not currently represent a widespread epizootic or epidemic threat on a global scale.
The preservation and amplification of lyssavirus strains within their natural biocenoses depend on their sustained circulation among reservoir host populations. Furthermore, the epizootic dynamics of rabies are shaped by the landscape and climatic conditions of ecologically favorable yet epidemiologically high-risk ecosystems [20]. Analyzing the spatiotemporal dynamics of animal rabies incidence across natural and agricultural zones enables the identification of high-risk areas and key ecological factors influencing epizootic stability in different ecosystems [21].
MATERIALS AND METHODS
Using Microsoft Access (relational database management system www.microsoft.com), data from reports of the Veterinary Department under the Ministry of Agriculture of the Russian Federation on rabies outbreaks and rabies vaccination among wild animals were aggregated. The database was structured to categorize all records by time period, administrative region, and affected animal species. All epizootiological data were georeferenced to administrative regions and cross-referenced with Russia’s natural and agricultural zoning data [22]. Graphical visualization and statistical data were processed using Microsoft Excel Data Analysis ToolPak (www.microsoft.com).
For spatial analysis, records of all rabies outbreaks were georeferenced and linked to unique identifier (ID) codes from the attribute tables of administrative boundary vector layers of the Russian Federation. This enabled the creation of thematic vector layers incorporating epizootiological data within the GIS project. The GIS project was constructed using QGIS Desktop platform (www.qgis.org).
STUDY RESULTS
Analysis of 35 years of epizootiological monitoring data reveals continuous temporal variation in rabies epizootic indicators across the Russian Federation. These fluctuations affected all key epidemiological parameters, particularly annual incidence rates and the relative contribution of different animal species to epizootic transmission.
As shown in Figure 1, the most pronounced incidence fluctuations occurred in wildlife populations. Until 1997, the number of reported rabies cases among wild animals was significantly lower than the number of cases among livestock and pets, which can be explained by the lack of active and passive monitoring tests among wildlife. Over the following decade, diagnostic methods improved and the number of tests among wild animals increased. During this period, in the absence of oral vaccination for wild carnivores, the epizootic process exhibited distinct multi-year cycles characterized by 2–4 year intervals between peak incidence rates. This pattern reflects autocorrelation in the epizootic process, wherein pathogen-induced mortality affects reservoir host population density.
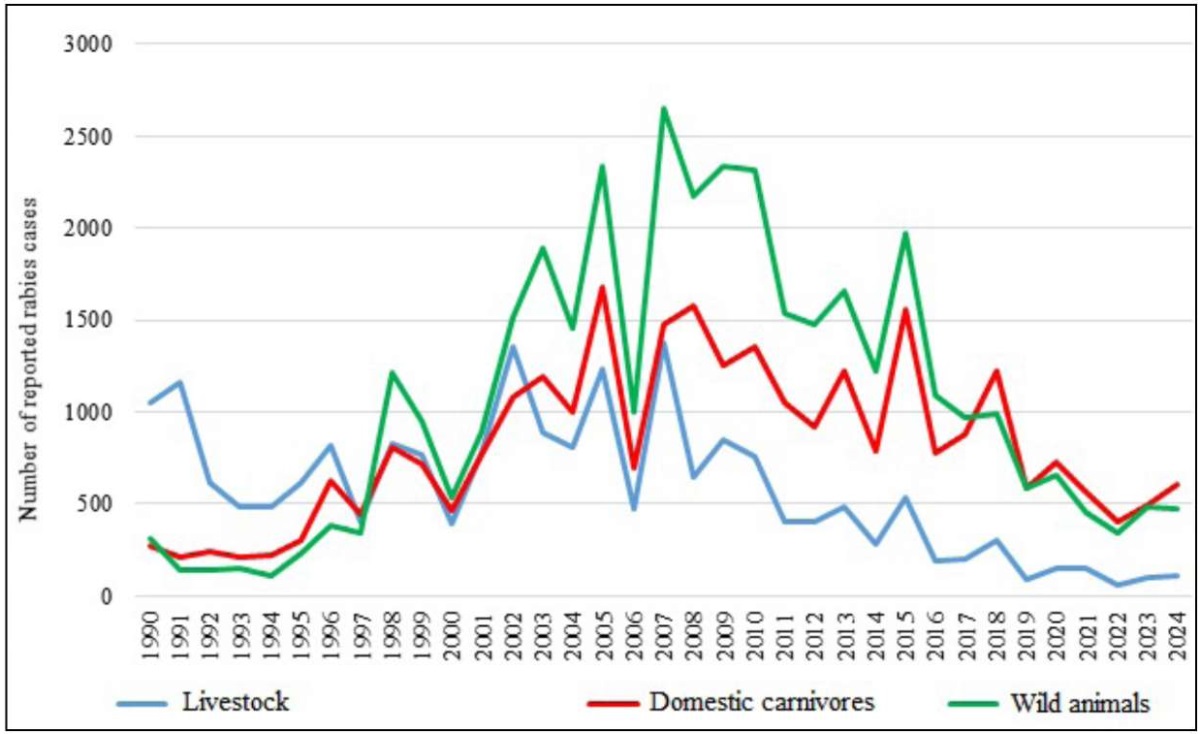
Fig. 1. Annual rabies incidence curves in the major species groups
In the middle of the 35-year period under review, the use of rabies oral vaccines of wild carnivores was initiated. Following the intensification of wildlife rabies vaccination efforts, both a progressive decline in disease incidence and stabilization of epizootic cycle amplitudes were observed [9].
Comparison of the incidence curves in Figure 1 reveals that the rabies incidence in domestic carnivores and farm animals directly correlates with the number of cases in wild animals. This correlation is evident within epizootic cycles, though long-term trends. Following 2007, incidence rates declined more substantially in livestock populations compared to domestic carnivores. The technological modernization of livestock production in recent decades has coincided with a reduction in total herd sizes, including grazing animal populations. The total number of cattle, according to Rosstat, was: in 1990 – 57,043.0 thousand animals; in 2007 – 21,501.6 thousand animals; in 2023 – 17,068.2 thousand animals (as of the end of the year). While farm animal populations have remained stable or decreased in some areas, the number of cats and dogs, particularly in rural areas and small towns – has increased, raising the potential for wildlife interactions [23].
The past decade to decade-and-a-half has represented a period of relative stability in ecological and epidemiological drivers influencing rabies epizootic dynamics. During this period in the Russian Federation, oral rabies vaccination campaigns targeting wild predators have been conducted on a regular basis. The minimum amount of oral vaccine used per year was in 2014–2015 (5.5 and 5.0 million doses, respectively). In other years, this figure ranged from 12.0 million doses (2013) to 26.4 million doses (2020). On average, about 16 million doses of oral rabies vaccine were used annually. That is why, 2013–2024 period was chosen for the study of the rabies epizootic process patterns.
It was found that rabies was most often detected among foxes, dogs, cats, cattle and raccoon dogs (Fig. 2).
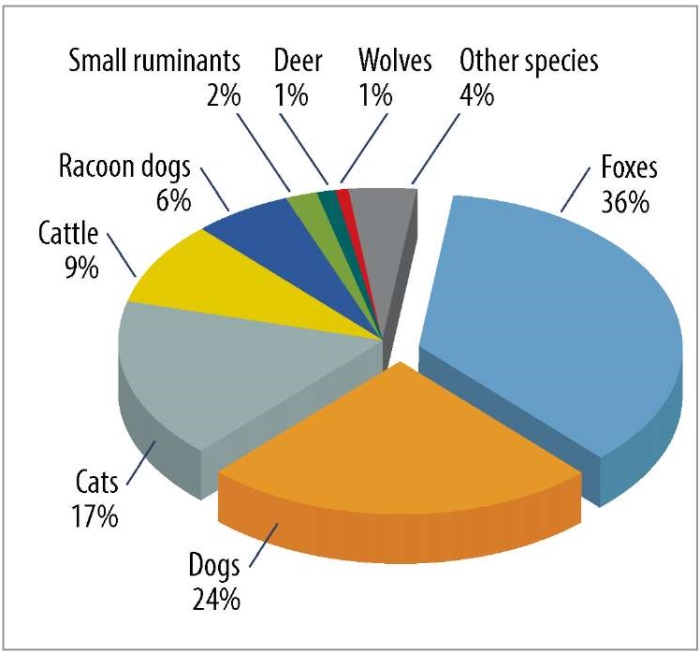
Fig. 2. Percentage of rabies cases by animal species in 2013–2024
Contemporary rabies epizootics in the Russian Federation are characterized not only by multi-annual incidence cycles but also by distinct seasonal patterns, largely determined by the biological and behavioral characteristics of the red fox as the principal viral reservoir and vector.
Rabies incidence reaches its annual nadir in early summer, corresponding to the fox cub-rearing period when adult vulpine hosts exhibit minimal migratory activity and restricted home ranges. At the end of summer, young foxes become more active, dispersing from their birth areas to establish their own territories, thus inducing an autumn increase in morbidity. The sharpest increase in rabies cases among foxes typically occurs in March, due to the increased activity and interactions between animals during the winter mating season (Fig. 3).
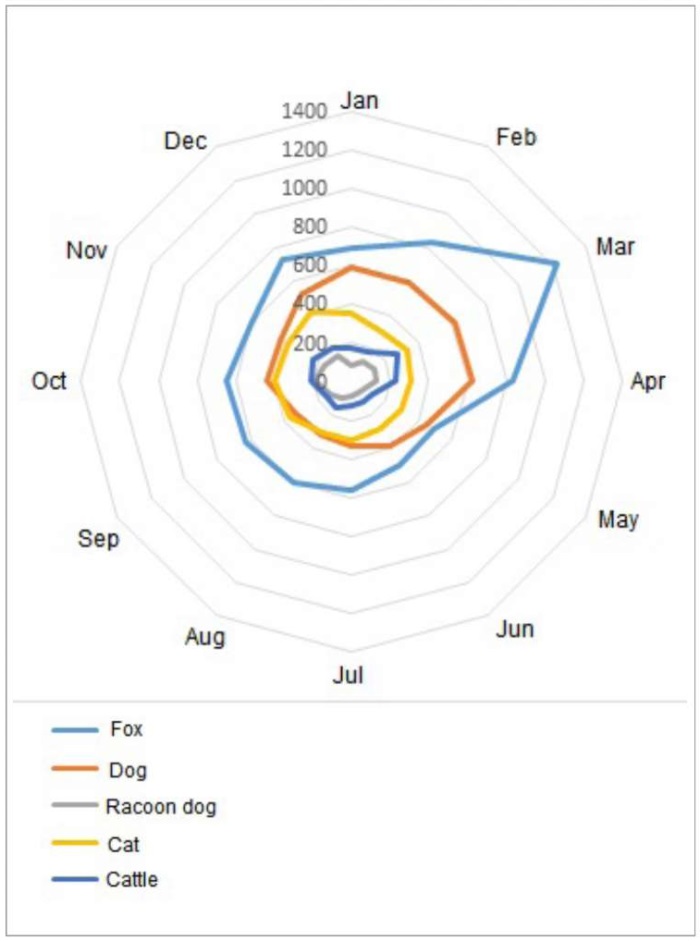
Fig. 3. Dynamics of seasonal rabies incidence in animals involved in the rabies process (according to monitoring data for 2013–2024)
An analysis of data on rabies-infected localities over this period shows that the current rabies distribution area involves most of the Russian Federation regions. Excluding the four new regions, rabies cases have been reported in 78 regions of the country over the past 12 years, and rabies outbreaks have been reported annually in 39 of them. The high incidence and spatial density of rabies outbreaks in the new regions, based on the observation data for the last two years, makes it possible to classify these territories as a high-risk zone.
Over the 12-year period, 30 regions of the country can be classified as the most severely infected, with over 80% of rabies outbreaks reported (Table 1).
Table 1
The subjects of the Russian Federation with the highest rabies incidence in 2013–2024
|
Federal District |
Russian Federation Subject |
Rabies cases reported |
Percentage relationship between the two periods, % |
||
|
In total |
2013–2018 |
2019–2024 |
|||
|
Central |
Moscow Oblast. |
1,501 |
1,221 |
280 |
23 |
|
Volga |
Saratov Oblast |
1,229 |
842 |
387 |
46 |
|
Central |
Belgorod Oblast |
1,220 |
996 |
224 |
22 |
|
Central |
Lipetsk Oblast |
931 |
852 |
79 |
9 |
|
Volga |
Republic of Tatarstan |
811 |
719 |
92 |
13 |
|
Ural |
Chelyabinsk Oblast |
797 |
437 |
360 |
82 |
|
Volga |
Penza Oblast |
791 |
470 |
321 |
68 |
|
Central |
Voronezh Oblast |
734 |
522 |
212 |
41 |
|
Central |
Tambov Oblast |
733 |
493 |
240 |
49 |
|
Central |
Vladimir Oblast |
684 |
443 |
241 |
54 |
|
Southern |
Volgograd Oblast |
680 |
478 |
202 |
42 |
|
Central |
Smolensk Oblast |
665 |
371 |
294 |
79 |
|
Central |
Tver Oblast |
656 |
501 |
155 |
31 |
|
Volga |
Republic of Udmurtia |
646 |
524 |
122 |
23 |
|
Volga |
Orenburg Oblast |
565 |
453 |
112 |
25 |
|
Volga |
Nizhny Novgorod Oblast |
563 |
309 |
254 |
82 |
|
Volga |
Samara Oblast |
530 |
255 |
275 |
108 |
|
Central |
Bryansk Oblast |
509 |
434 |
75 |
17 |
|
Central |
Yaroslavl Oblast |
507 |
411 |
96 |
23 |
|
Ural |
Sverdlovsk Oblast |
466 |
321 |
145 |
45 |
|
Ural |
Tyumen Oblast |
425 |
214 |
211 |
99 |
|
Central |
Tula Oblast |
414 |
365 |
49 |
13 |
|
Central |
Ryazan Oblast |
382 |
245 |
137 |
56 |
|
Volga |
Kirov Oblast |
370 |
315 |
55 |
17 |
|
Siberian |
Krasnoyarsk Krai |
309 |
110 |
199 |
181 |
|
Siberian |
Novosibirsk Oblast |
298 |
184 |
114 |
62 |
|
Central |
Kursk Oblast |
295 |
257 |
38 |
15 |
|
Siberian |
Republic of Khakassia |
287 |
156 |
131 |
84 |
|
Southern |
Astrakhan Oblast |
248 |
180 |
68 |
38 |
|
Central |
Kaluga Oblast |
238 |
150 |
88 |
59 |
The highest incidence rates over the 12 years were reported in the Central and Volga Federal Districts. Herewith, a comparative analysis of six-year intervals in these regions demonstrated substantial decline in rabies incidence.
As evidenced by Table 1, the decline in disease incidence occurred asynchronously across regions, with some areas showing minimal change or even increased case numbers.
The current rabies distribution area is mainly associated with warmer regions of the Russian Federation. A negligible number of cases was reported in the biomes of the northern territories.
A characteristic geographical pattern shows that rabies outbreaks have lower spatial density in the subzones of the northern and middle taiga regions. The limited monitoring data from boreal forests reflects both low human population density and inherently low rabies prevalence, as evidenced by consistently minimal cases among domestic carnivores – suggesting ecological factors naturally limit disease transmission in these ecosystems. Rabies epizootics in boreal forests naturally attenuate due to three key ecological factors: low predator population densities resulting from limited prey availability, landscape barriers that restrict host movement, and winter snow cover that impedes migratory transmission.
Comparative spatial analysis of rabies outbreaks between 2013–2018 and 2019–2024 revealed decreased outbreak density (cases per unit area) while the geographic extent of affected regions remained stable (Fig. 4).
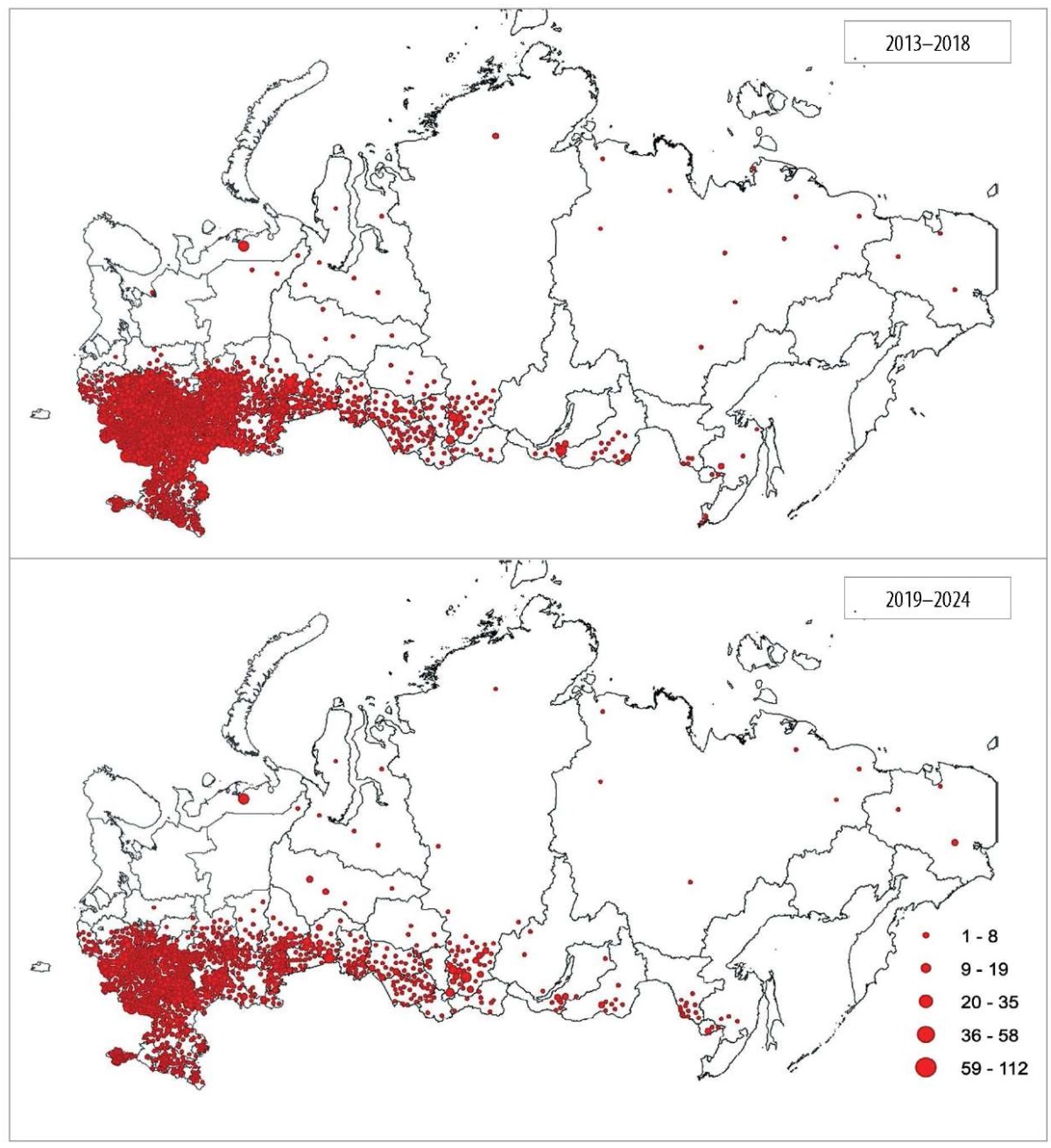
Fig. 4. Location and number of rabies outbreaks reported in the administrative regions of the Russian Federation in 2013–2018 and 2019–2024
The observed spatial heterogeneity in rabies outbreak distribution reflects environmental factors influencing wild predator ecology – particularly those affecting host abundance and dispersal patterns. Consequently, current epizootic risk zones show a strong correlation with agroecological provinces and zones.
Between 2013 and 2024, rabies was detected in 42 agroecological provinces of Russia, with over 50% of cases concentrated in two high-risk zones: the Middle Russian forest-steppe and the Southern Taiga Forest ecosystems (Fig. 5).
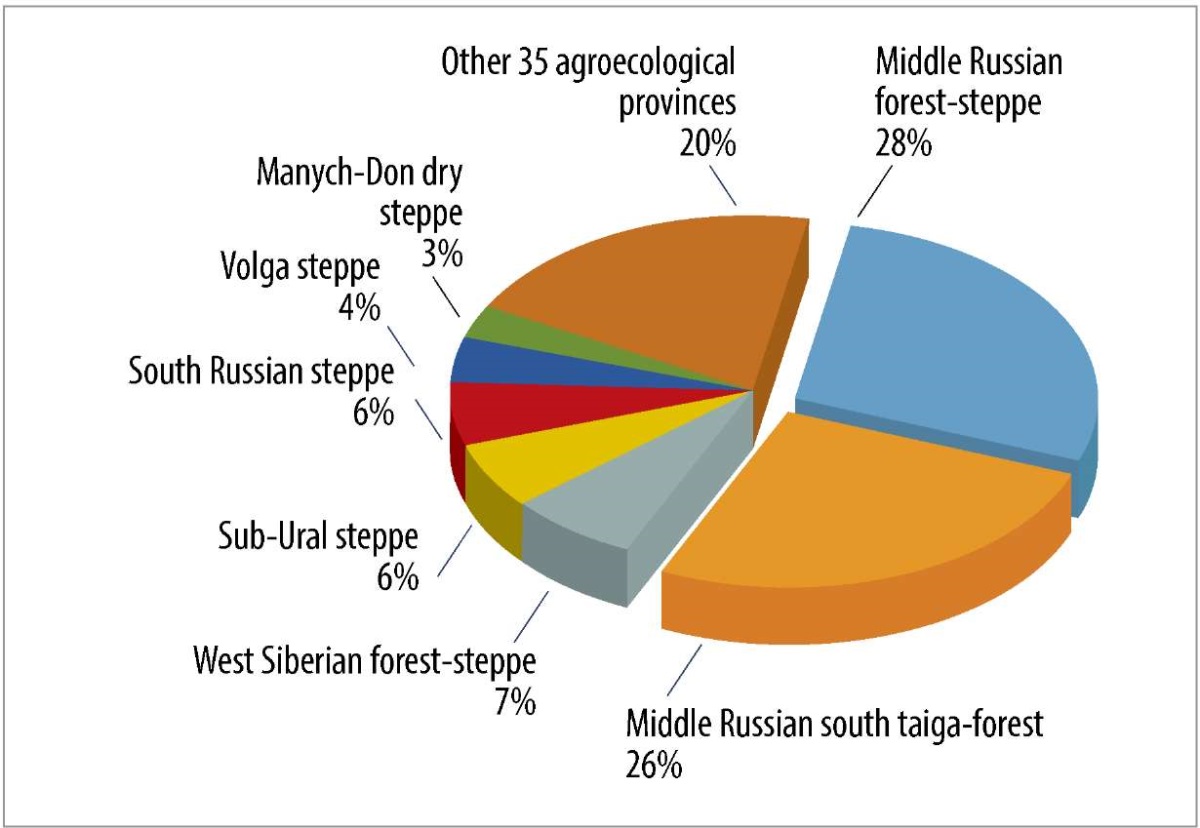
Fig. 5. Distribution of rabies outbreaks reported in 2013–2024 by agroecological provinces of the Russian Federation
The association between rabies occurrence and natural ecosystem characteristics influences both the spatial distribution of outbreaks across administrative units and regional variations in affected animal species (Table 2).
Table 2
Rabies infected animals of various groups by territories (according to monitoring data for 2013–2024)
|
Federal District |
Wild animals |
Pet animals |
Farm animals |
|||
|
Total animals |
Infected, % |
Total animals |
Infected, % |
In total |
Infected, % |
|
|
North-Western |
218 |
60 |
61 |
17 |
85 |
23 |
|
Central |
4,909 |
49 |
4,410 |
44 |
711 |
7 |
|
Volga |
2,771 |
43 |
2,823 |
44 |
876 |
14 |
|
Southern |
362 |
24 |
935 |
61 |
243 |
16 |
|
North Caucasian |
61 |
11 |
290 |
51 |
223 |
39 |
|
Ural |
1,244 |
60 |
559 |
27 |
259 |
13 |
|
Siberian |
928 |
53 |
359 |
21 |
448 |
26 |
|
Far Eastern |
110 |
48 |
61 |
27 |
57 |
25 |
The spatial distribution of rabies cases varies significantly among different species. In colder regions, rabies cases predominantly occur in wild animals, whereas in warmer regions, domestic carnivores represent the main epizootiological risk group. This pattern can be attributed to the intensive interpenetration of natural and anthropogenic landscapes in southern regions, where human activity and ecosystems are deeply intertwined.
The lowest rabies incidence among foxes during 2013–2024 was reported in the Caucasian Mountain natural area. Of the 192 recorded rabies cases, only 7 (4%) occurred in foxes. Furthermore, this area shows no seasonal peak in animal rabies incidence during March, which is characteristic of fox rabies in lowland biomes.
DISCUSSION AND CONCLUSIONS
Rabies persistence in the Russian Federation is maintained by the virus’s reservoir in wild fox populations. Between 2013 and 2024, foxes accounted for 36% of all reported rabies cases. The most extensive and infected zone comprises two agroecological provinces: the Middle Russian forest-steppe and Middle Russian south taiga-forest. The ecological conditions of the East European Plain’s forest-steppe zone and adjacent southern taiga (mixed and deciduous forests) support robust canid predator populations through abundant food resources while facilitating widespread rabies transmission across the region. The synergistic effect of these ecological and epidemiological factors sustains the epizootic cycle, maintaining consistently high rabies incidence rates across the region.
Domestic animals – particularly dogs, cats, and cattle – collectively represent the most frequently reported rabies cases among other species, collectively comprising approximately 50% of total disease incidence. All cases of infection among these species can be regarded as a side effect of the sylvatic cycle spillover. While dogs account for approximately 24% of animal rabies cases in Russia, the question of a sustained urban rabies cycle (urban rabies) in the Russian Federation remains complex. However, with a well-developed veterinary service and a government-funded rabies vaccination program, the probability of the virus establishing stable, independent circulation in the dog population is extremely low. Due to their behavior, domestic carnivores are the most common victims of rabid foxes invading settlements. As such, they serve as clear sentinels for sylvatic rabies outbreaks, especially in areas with insufficient disease monitoring. However, in some territories, primarily in the North Caucasus, the epidemiology of rabies exhibits distinct characteristics that sharply contrast with the rest of the country. In the Caucasian Mountain natural area, rabies incidence in foxes is reported as 4%, with no observed seasonal increase in March. This evidence supports the hypothesis of persistent, independent canine rabies circulation in localized zones [24]. This possibility is supported by studies demonstrating the genetic isolation of Caucasus-region rabies virus strains. Furthermore, the region’s geographical proximity to the canine rabies enzootic area of Asia Minor provides a plausible explanation for this distinct epidemiological pattern [25][26].
Oral vaccination campaigns targeting wild predators over the past 15 years have driven a significant reduction in the overall incidence of animal rabies in the Russian Federation [27]. The most pronounced decline occurred in fox population. During this period, several short-term increases in incidence interrupted the overall downward trend. The most significant peak in animal rabies incidence occurred in 2015, resulting from a dramatic reduction in the oral vaccination of wild predators during the preceding year (2014–2015). The decline in rabies incidence among domestic dogs and cats has been less pronounced in recent years, which is likely attributable to their actively growing populations.
A comparative spatial analysis of rabies outbreaks over the last two six-year periods reveals that, despite the overall incidence decline, the virus persists in historically endemic areas and has not been eliminated. While this strategy significantly reduces epizootic and epidemiological risks, any reduction in the oral vaccination of wild predators would likely lead to a rapid deterioration of the rabies situation. To date, Russia’s successful rabies eradication using oral vaccine baits has been confined to regions that are either geographically isolated from the main disease distribution area, like Kaliningrad Oblast, or located on its periphery, such as Leningrad Oblast and the Republic of Karelia [28][29].
The current epizootic situation necessitates a new disease control strategy focused on containing and reducing the disease distribution area. The scientific foundation for territorial rabies eradication programs should be based on the principles of systemic epizootology. This discipline analyzes pathogen spread within the context of local ecosystems and the impact of existing disease control measures [21].
Contribution of the authors: Gulyukin A. M. – conceptualization and study design, review and validation of the text and results, final editing of the manuscript; Shabeykin A. A. – conceptualization and study design, spatial and statistical data analysis, preparation of the manuscript draft, review and validation of the text and results, text formatting and preparation.
Вклад авторов: Гулюкин А. М. – концепция и дизайн исследования, рецензирование текста и полученных результатов, финальное редактирование рукописи; Шабейкин А. А. – концепция и дизайн исследования, проведение пространственного и статистического анализа данных, подготовка рукописи, рецензирование текста и полученных результатов, оформление текста.
References
1. Epidemiology of Rabies. Rabies – Bulletin – Europe. WHO Collaborating Centre for Rabies Surveillance & Research. https://who-rabies-bulletin.org/site-page/epidemiology-rabies
2. Genus: Lyssavirus. In: Rhabdoviridae. International Committee on Taxonomy of Viruses. https://ictv.global/report/chapter/rhabdoviridae/rhabdoviridae/lyssavirus
3. Rupprecht C. E., Turmelle A., Kuzmin I. V. A perspective on lyssavirus emergence and perpetuation. Current Opinion in Virology. 2011; 1 (6): 662–670. https://doi.org/10.1016/j.coviro.2011.10.014
4. Badrane H., Tordo N. Host switching in Lyssavirus history from the Chiroptera to the Carnivora orders. Journal of Virology. 2001; 75 (17): 8096–8104. https://doi.org/10.1128/jvi.75.17.8096-8104.2001
5. Kuzmin I. V., Shi M., Orciari L. A., Yager P. A., Velasco-Villa A., Kuzmina N. A., et al. Molecular inferences suggest multiple host shifts of rabies viruses from bats to mesocarnivores in Arizona during 2001–2009. PLoS Pathogens. 2012; 8 (6):e1002786. https://doi.org/10.1371/journal.ppat.1002786
6. Marston D. A., Banyard A. C., McElhinney L. M., Freuling C. M., Finke S., de Lamballerie X., et al. The lyssavirus host-specificity conundrum-rabies virus – the exception not the rule. Current Opinion in Virology. 2018; 28: 68–73. https://doi.org/10.1016/j.coviro.2017.11.007
7. Makarov V. V., Gulyukin A. M., Gulyukin M. I. Rabies: Natural History at Centuries Boundary. Moscow: ZooVetKniga; 2015. 121 р. (in Russ.)
8. Metlin A. Ye. Modern aspects of Lyssavirus classification. Veterinary Science Today. 2017; (3): 52–57. https://elibrary.ru/zigafp (in Russ.)
9. Rupprecht C. E., Hanlon C. A., Slate D. Oral vaccination of wildlife against rabies: opportunities and challenges in prevention and control. Developments in Biologicals. 2004; 119: 173–184. https://pubmed.ncbi.nlm.nih.gov/15742629
10. Gruzdev K. N., Metlin A. Ye. Animal Rabies. 2nd ed., revised and expanded. Vladimir: Federal Centre for Animal Health; 2022. 442 p. (in Russ.)
11. Cherkasskiy B. L. Epidemiology and Prevention of Rabies. Moscow: Medicine; 1985. 287 p. (in Russ.)
12. Poleshchuk E. M., Sidorov G. N., Gribencha S. V. A summary of the data about antigenic and genetic diversity of rabies virus circulating in the terrestrial mammals in Russia. Problems of Virology. 2013; 58 (3): 9–16. https://elibrary.ru/pzxttn (in Russ.)
13. Bourhy H., Kissi B., Audry L., Smreczak M., Sadkowska-Todys M., Kulonen K., et al. Ecology and evolution of rabies virus in Europe. Journal of General Virology. 1999; 80 (10): 2545–2557. https://doi.org/10.1099/0022-1317-80-10-2545
14. Makarov V. V., Sukharev O. I., Gulyukin A. M., Sokolov M. N., Litvinov O. B. Statistical analyses morbidity racoon dogs rabies. Veterinariya. 2009; (6): 20–25. https://elibrary.ru/kwzdoz (in Russ.)
15. Matouch O. The rabies situation in Eastern Europe. Developments in Biologicals. 2008; 131: 27–35. https://pubmed.ncbi.nlm.nih.gov/18634463
16. Gulyukin A. M., Shabeikyn A. A., Patrikeev V. V., Parshikova A. V., Tsaregradskiy P. Yu., Shabeykina M. V. Features of the epizootic process of rabies in the Eastern part of the European nosoareal. Veterinariya. 2022; (12): 15–21. https://doi.org/10.30896/0042-4846.2022.25.12.15-21 (in Russ.)
17. Botvinkin A. D., Poleschuk E. M., Kuzmin I. V., Borisova T. I., Gazaryan S. V., Yager P., Rupprecht C. E. Novel lyssaviruses isolated from bats in Russia. Emerging Infectious Diseases. 2003; 9 (12): 1623–1625. https://doi.org/10.3201/eid0912.030374
18. Speranskaya A. S., Samoilov A. E., Kaptelova V. V., Artyushin I. V., Simonova E. G., Shabeykin A. A., et al. Genome sequence of European bat 1 lyssavirus isolated from Eptesicus serotinus, which was caught near Voronezh city in late 2019. Molecular Diagnostics and Biosafety – 2020: Russian national scientific and practical conference with international participation (Moscow,October 6–8, 2020): conference proceedings. Moscow: Central Research Institute for Epidemiology; 2020; 251–252. https://elibrary.ru/yisewj (in Russ.)
19. Poleshchuk E. M., Tagakova D. N., Sidorov G. N., Orlova T. S., Gordeiko N. S., Kaisarov A. Zh. Lethal cases of lyssavirus encephalitis in humans after contact with bats in the Russian Far East in 2019–2021. Problems of Virology. 2023; 68 (1): 45–58. https://doi.org/10.36233/0507-4088-156
20. Shabejkin A. A., Gulyukin A. M., Parshikova A. V. Analysis of patterns in rabies epizootic process in the European part of the Russian Federation. Veterinaria i kormlenie. 2015; (1): 29–34. https://elibrary.ru/tgucgv (in Russ.)
21. Gulyukin A. M. The principles of building an informational and analytical systems for forecasting and modeling epizootological risks. Veterinariya. 2024; (9): 3–8. https://doi.org/10.30896/0042-4846.2024.27.9.03-08 (in Russ.)
22. Gaidamaka E. I., Rozov N. N., Sashko D. I., Bondarchuk N. P., Bulgakov D. S., Vadkovskaya N. N. et al. Natural and Agricultural Zoning of the Land Fund of the USSR. Ed. by A. N. Kashtanov. Moscow: Kolos; 1983. 336 p. (in Russ.)
23. The number of pets in the Russian Federation has increased by 11% in three years. TASS Russian News Agency. April 10, 2024. https://tass.ru/novosti-partnerov/20499757 (in Russ.)
24. Cherkasskii B. L., Knop A. G., Vedernikov V. A., Sedov V. A., Khaĭrushev A. E., Chernichenko S. A. The epidemiology and epizootiology of rabies on the territory of the former USSR. Journal of Microbiology, Epidemiology and Immunobiology. 1995; 72 (1): 21–26. https://pubmed.ncbi.nlm.nih.gov/7778368 (in Russ.)
25. Marston D. A., Horton D. L., Nunez J., Ellis R. J., Orton R. J., Johnson N., et al. Genetic analysis of a rabies virus host shift event reveals within-host viral dynamics in a new host. Virus Evolution. 2017; 3 (2):vex038. https://doi.org/10.1093/ve/vex038
26. Metlin A. E., Rybakov S., Gruzdev K., Neuvonen E., Huovilainen A. Genetic heterogeneity of Russian, Estonian and Finnish field rabies viruses. Archives of Virology. 2007; 152 (9): 1645–1654. https://doi.org/10.1007/s00705-007-1001-6
27. Paroshin A. V., Voskresensky S. B., Gruzdev K. N., Chernyshova E. V. Rabies situation in the Moscow oblast in 2011–2023 and the role of oral vaccination of wild carnivores against rabies. Veterinary Science Today. 2024; 13 (3): 214–222. https://doi.org/10.29326/2304-196X-2024-13-3-214-222
28. Ivanov A. V., Khismatullina N. A., Petrova T. P., Gulyukin A. M., Shabeikin A. A., Karimov M. M., et al. Rabies epizootic situation in the Kaliningrad Region. Veterinariya. 2015; (4): 9–13. https://elibrary.ru/tolysd (in Russ.)
29. Fogel L. S., Gruzdev K. N., Krotov L. N., Danko Yu. Yu. The practice of creating buffer zones in anti-epizootic measures for rabies using the example of the border zone with Finland. Legal Regulation in Veterinary Medicine. 2024; (1): 38–41. https://doi.org/10.52419/issn2782-6252.2024.1.38 (in Russ.)
About the Authors
A. M. GulyukinRussian Federation
Alexey M. Gulyukin - Dr. Sci. (Veterinary Medicine), Corresponding Member of the Russian Academy of Sciences, Director of Federal Scientific Centre VIEV.
24/1 Ryazansky prospekt, Moscow 109428
A. A. Shabeykin
Russian Federation
Alexander A. Shabeykin - Dr. Sci. (Veterinary Medicine), Head of the Laboratory of General Epizootology, Federal Scientific Centre VIEV.
24/1 Ryazansky prospekt, Moscow 109428
Review
For citations:
Gulyukin A.M., Shabeykin A.A. Rabies in the Russian Federation: A 35-year review of trends, patterns, and influencing factors. Veterinary Science Today. 2025;14(3):232-240. https://doi.org/10.29326/2304-196X-2025-14-3-232-240



































