Scroll to:
Identification of Escherichia coli, Escherichia albertii, Proteus vulgaris biofilms detected in poultry with respiratory and gastrointestinal diseases
https://doi.org/10.29326/2304-196X-2025-14-2-186-193
Abstract
Introduction. When the body resistance-associated compensatory mechanisms are impaired or evolutionarily developed microbiocenoses are changed the quorum sensing signaling molecules facilitates excessive growth of pathogenic microorganisms. Antibacterial potential of inhibitors of intercellular communication molecule synthesis is achieved through reducing the microorganism adhesion and, consequently, in vivo and in vitro contamination.
Objective. Study of the dynamics of morphometric and densitometric parameters of biofilms formed by Escherichia coli, Escherichia albertii, Proteus vulgaris isolates identified in poultry with respiratory and gastrointestinal diseases.
Materials and methods. Dynamics of the biofilms formed by reference strains and isolates recovered from pathological samples from ROSS-308 chickens at the age of 40–42 weeks (n = 20) were studied. The sample optical densities were determined using Immunochem-2100 photometric analyzer (HTI, USA), wavelength 580 nm (OD580). Morphometric parameters were recorded at ≥ 90.0% reliable frequency in the field of view of Н604 Trinocular Unico optical microscope (United Products & Instruments Inc., USA) and Hitachi TM3030 Plus scanning electron microscope (Hitachi, Japan).
Results. Escherichia coli, Escherichia albertii, and Proteus vulgaris were isolated from pathological samples from the poultry with catarrhal hemorrhagic aerosacculitis, hemorrhagic enteritis, fibrinous polyserositis and splenomegaly signs and then identified. Direct correlations (r = 0.91) between morphometric and densitometric parameters depending on the cultivation time were established. Cells with defective cell walls, spheroplasts, needle-like and giant structures as well as revertant cells dominated during heterogeneous population dispersion.
Conclusion. General patterns of the heterogeneous microorganism population development are mediated by adhesion, synthesis of exocellular molecules, intensive cell proliferation and differentiation depending on the cell cycle stage.
Keywords
For citations:
Lenchenko E.M., Ponomarev V.V., Sachivkina N.P. Identification of Escherichia coli, Escherichia albertii, Proteus vulgaris biofilms detected in poultry with respiratory and gastrointestinal diseases. Veterinary Science Today. 2025;14(2):186-193. https://doi.org/10.29326/2304-196X-2025-14-2-186-193
INTRODUCTION
In view of globalization of the spread of new and variants of known nosological forms characterized by high epidemiological potential, there is a statistically significant trend to increase in incidence of the infections caused by antibiotic-resistant bacteria of the order Enterobacterales [1-4]. Due to their multidrug resistance, these bacteria are classified to the first category of critical priority level for research according to the WHO Bacterial Priority Pathogens List (2024) [5].
Clinical Escherichia coli isolates identified in humans with septicemia, neonatal meningitis, and urologic disorders are genetically similar and share common virulence genes with avian pathogenic E. coli (APEC) [6][7].
High population density in limited areas, keeping animals of the same species and age in the holding, use of antibiotics as well as frequent changes in the vaccination schedule including use of vaccines based on “hot” and variant strains contribute to the wide spread of infectious diseases [8]. According to the veterinary reports, colibacillosis is registered everywhere and responsible for significant economic losses [9][10]. In poultry with systemic infection, the dominance of E. coli as an etiological agent ranges from 50.7 to 100% depending on the disease situation on commercial poultry farms of various types as well as family-operated and backyard farms [11][12]. Increasing resistance of APEC to different classes of antibiotics, including socially important antibiotics (β-lactams, colistin, and carbapenems) is a marker of multiple APEC resistance [13-16].
E. coli pathogenic properties are accounted for virulence factors encoded by chromosomal, plasmid genes and chromosome-integrated bacteriophages [17][18]. When intestinal compensatory mechanisms of mucociliary clearance and colonization resistance are impaired and microbiocenosis quantitative and species composition are changed, representation of the quorum sensing (QS) signalling molecules contributes to the excessive growth of pathogenic microorganisms [19]. The therapeutic and disinfecting effect of QS inhibitors owing to blocking the intercellular communication molecules synthesis enables reducing the adhesion of microorganisms and, consequently, level of contamination in vivo and in vitro [20][21].
Studies of the etiological factors of respiratory and gastrointestinal diseases in poultry are of priority for identification of pathogenetical factors of initiation, development and outcome of the avian infectious pathology characterized by pathogenic enterobacteria excessive growth and dissemination. Study of the general patterns of multilevel algorithms for differentiation of heterogeneous population including viable uncultivated cells will facilitate optimization of the long-term retrospective identification of ubiquitous bacteria as well as development of methods for biofilm eradication in the future.
The aim of the work is to study the dynamics of morphometric and densitometric parameters of biofilms of E. coli, Escherichia albertii, Proteus vulgaris isolates identified in poultry with respiratory and gastrointestinal diseases.
MATERIALS AND METHODS
Strains. Isolates recovered in pathological samples collected from ROSS-308 cross chickens at the age of 40–42 weeks (n = 20) were used for tests. Reference Escherichia coli strain (ATCC 25922) from the Collection of the Tarasevich State Research Institute for Standardization and Control of Biological Products (Moscow) was used as a control [22].
Nutrient media. The following nutrient media were used: Endo medium, bismuth-sulfite agar (BSA; HiMedia, India), meat-peptone broth (MPB), meat-peptone agar (MPA), Hiss medium, Olkenitsky’s medium, Simmons’ citrate agar (State Research Center for Applied Microbiology, Russia), Tryptone Bile X-glucuronide agar, Chromocult® coliform agar (Merck, Germany).
Test system. The following test systems were used: Paper indicator systems for the microorganism identification; kit No. 2 for Enterobacteriaceae genus and species differentiation (Microgen, Russia); Boichemical plate for enterobacteria identification (Diagnostic Systems, Russia); ENTERO-Rapid 24, NEFERMtest 24 (Erba Lachema s.r.o., Czech Republic).
Postmortem examination. Dead chickens (n = 20) submitted to the Belgorod Branch of the Federal Centre for Animal Health for bacteriological examination from poultry farms located in the Central Black Earth region of the Russian Federation were subjected to postmortem examination (necropsy). The tests were performed in accordance with the Methodical guidelines for pathomorphological diagnosis of animal, avian, and fish diseases in veterinary laboratories: approved by the Veterinary Department of the Ministry of Agriculture of the Russian Federation on 11 September 2000, No. 13-7-2/2137 [23]. Postmortem examination was carried using common methods and taking into account the chicken anatomical and topographic features [24-26].
Microbiological tests were carried out in accordance with the Methodical guidelines for bacteriological diagnosis of mixed intestinal infection in young animals caused by pathogenic enterobacteria, approved by the Veterinary Department of the Ministry of Agriculture of the Russian Federation on 11 October 1999, No. 13-7-2/1759; Methodical guidelines for bacteriological diagnosis of animal colibacillosis (escherichiosis), approved by the Veterinary Department of the Ministry of Agriculture of the Russian Federation on 27 July 2000, No. 13-7-2/2117; Methodological guidelines for Isolation of bacteria from the animal gastrointestinal tract and identification thereof, approved by the Veterinary Department of the Ministry of Agriculture of the Russian Federation on 11 May 2004, No. 13-5-02/ 1043 [27-29].
The authors confirm compliance with institutional and national standards in accordance with the Consensus Author Guidelines for Animal Use (IAVES, July 23, 2010). The test protocol was approved by the Ethics Committee of the RUDN University, Moscow, Russian Federation (Protocol No. 9a/3 of 8 October 2024).
Small intestine and caecum contents were examined for microorganism quantification. Test samples weighing 1.0 g were placed in test tubes and of 0.85% sodium chloride solution was added to the tubes, 9.0 cm3 per tube. Diagnostically significant dilutions were made, then 0.1 mL of the test sample was inoculated onto differential media.
Test pathological sample (heart with ligated vessels, lungs, tubular bone, liver with gall bladder, spleen) was put with a Pasteur pipette to the middle part of a Petri dish and evenly distributed with a glass spatula. For small intestine examination, its contents were removed, mucous membrane was carefully scraped off using a scarifying cone of a Pasteur pipette and the material was inoculated onto the medium. In order to avoid the swarming bacteria growth, Endo medium surface was irrigated with 96% ethanol (1–2 cm3) before material inoculation. Microorganisms were cultured at (37 ± 1) °C for (24 ± 1) hours and (48 ± 1) hours. To isolate pure Proteus spp. cultures, the materials were inoculated according to Shukevich method in condensed fluid of freshly slanted MPA and cultivated at (37 ± 1) °C for (24 ± 1) hours. When the growth was observed, the microorganisms were transferred to the BSA medium and cultured at (37 ± 1) °C for (24 ± 1) and (48 ± 1) hours [24][27][28].
For species identification, three species-characteristic colonies of microorganisms were transferred into tubes with slanted MPA and cultured at (37 ± 1) °C for (24 ± 1) hours. The microorganisms were tested for their morphological, cultural, and biochemical properties using common methods [1][27][28][29].
Biofilm tests. For densitometric tests, the test samples were added to wells of a 96-well plate (Medpolymer OJSC, Russia) and cultured at (37 ± 1) °C under static aerobic conditions for 6, 18, 24, 48 hours. After the specified time, the fluid was removed from the plate wells, and the sediment was washed with 200 µL of phosphate buffer solution (pH 7.2) three times. At each washing stage, the plate contents were stirred at 2,000 rpm for 10 minutes using MixMate vortex shaker (Eppendorf, Germany). The samples were fixed with 96% ethanol for 15 minutes and dried at (37 ± 1) °C for 20 minutes. Then, 0.5% crystalline violet solution (HiMedia, India) was added to the wells and the plates were cultured at (37 ± 1) °C for 5 minutes. The well contents were removed, the wells were washed with 200 µL of phosphate buffer solution (pH 7.3) three times, and dried. The dye was eluted with 200 µL of 96% ethanol for 30 minutes [30][31]. The optical densities of the test samples were determined using an ImmunoChem-2100 photometric analyzer (HTI, USA) at a wavelength of 580 nm (OD580).
For morphometric tests, the preparations were fixed with ethanol-ether mixture (1:1) for 10 minutes and stained with an aqueous gentian violet solution (1:2,000) and Gram stained (BioVitrum, Russia). For scanning electron microscopy, the preparations were fixed with 25% glutaraldehyde solution vapours for 8 hours, and then with 1% osmium tetroxide solution vapours for 4 hours. The test samples were thickened with ethanol at increasing concentration: 30, 50, 96, 100%. Then, test samples were exposed to gold ions using a Q150T ES device (Quorum Technologies Ltd., Great Britain). Morphometric parameters were recorded at significant ≥ 90.0% frequency in the field of view of the H604 Trinocular Unico optical microscope (United Products & Instruments Inc., USA) and Hitachi TM3030 Plus scanning electron microscope (Hitachi, Japan).
Statistical analysis using the Student’s criterion was used for the test result processing; the results were considered reliable at p ≤ 0.05 [19].
RESULTS AND DISCUSSION
Postmortem examination. Postmortem examination of dead ROSS-308 chickens at the age of 40–42 weeks (n = 20) showed the following: the chicken feathers were dull and ruffled; the dead chickens were emaciated. Cyanosis of mucous membranes, uneven and extreme swelling of stomach, small intestine and caecum were detected. Multiple petechial and striated haemorrhages were found in the muscles and tracheal, stomach and intestinal mucosa. Acute congestive hyperemia of cardiovascular organs was characterized by blood vessel congestion, edematous fluid accumulation in loose connective tissue, red blood cell hemolysis. Catarrhal hemorrhagic aerosacculitis, splenomegaly, hemorrhagic enteritis and fibrinous polyserositis manifestations were detected (Fig. 1).
Detection and identification of microorganisms. The bacteria formed round glossy convex colonies with even edges, 1.5–2.5 mm in diameter when the test samples were inoculated onto differential nutrient media intended for primary identification.
On Endo medium, lactose fermenting microorganisms formed red colonies, some of which had a characteristic metallic glitter. The number of colonies grown onto media inoculated with small intestine and cecum contents was (1.43 ± 0.25) × 106 CFU/g; and (4.6 ± 0.32) × 107 CFU/g, respectively. Lactose-non-fermenting microorganisms were isolated from the chicken small intestine contents together with the specified bacteria; number of the pinkish colonies colourless in the centre was (0.85 ± 0.34) × 104 CFU/g (Fig. 2A).
When test samples were inoculated with Shukevich method in the condensed fluid of freshly slanted MPA, microorganism growth was observed. The cultures transferred from MPA to BSA medium formed dark-green colonies surrounded by zone of inhibition, the number of colonies was (0.77 ± 0.87) × 103 CFU/g (Fig. 2B).
Gram-negative, facultative anaerobic, oxidase-negative, and catalase-positive E. coli isolates were identified when pure cultures of the microorganisms isolated from the pathological samples of all tested chickens (100%) were tested for their morphological, tinctorial, and biochemical properties. E. coli monocultures were detected in small intestine content samples from 16 chickens (80%). E. albertii and P. vulgaris bacteria were detected together with E. coli in tested small intestine samples from 4 chickens (20%).
Morphological and densitometric parameters of biofilms. E. coli, E. albertii, and P. vulgaris isolates cultivated at (37 ± 1) °C for 6, 18, 24, 48 hours under static aerobic conditions showed common patterns for biofilm development regardless of the isolation origin. The changes in absolute values of tested sample optical density and the biofilm formation intensity are given in the Table.
Depending on cultivation duration, direct correlations (r = 0.91) were observed between densitometric parameter intensities and increased frequency of visualized bacterial coaggregation within the intercellular matrix.
The following stages of biofilm development were identified at representative ≥ 90.0% field of view of the microscope: adhesion, fixation, microcolony, growth, and dispersion. Adsorption and nonspecific adhesion of microorganisms to the tested substrate surface – glass owing to conditioning were detected at the initial stages of development. Moreover, at this stage, cells can either attach to the substrate surface or detach from it and return to planktonic phase of growth. Intermolecular interactions between specific microbial cell wall structures mediate irreversible adhesion and surface attachment. Once microorganisms were firmly attached to the substrate surface they promoted adhesion of subsequent cells. Cells exhibiting distinct morphologies and sizes but interconnected within an extracellular matrix were differentiated depending on the cell cycle stage (Fig. 3).
Clusters (aggregates, conglomerates) formed and grew owing to the binary division of bacteria during intensive proliferation of the cells synthesizing exocellular molecules. Rounded fluid-filled structures – canals serving for the population hydration were detected between clusters of orderly and at the same time multidirectionally arranged cells. Extracellular matrix exhibited progressive thickening correlating with both increased numbers of attached dividing cells and enhanced synthesis of exopolymeric components. The matrix components were differentiated based on the chemical composition when the cells were stained with metachromatic aniline dyes with properties: protein structures stained blue, polysaccharides stained pink (Fig. 4).
Mature three-dimensional heteromorphic biofilm becomes immobilized through QS-mediated intercellular communication driven by population expansion and extracellular matrix development. Dispersion of the heteromorphic population increased with prolongation of the cultivation time. Bacteria characteristic for L-transformation were detected together with the cells typical for the bacteria species. The following cells dominated: cells with defective cell walls, spheroplasts, needle-like and giant structures, as well as cells capable of reverting to their original phenotypic and metabolic state. The destruction, partial or complete autolysis of cells losing typical morphofunctional features (uncultivable cells) were accompanied by enhanced light refraction combined with decrease in the optical density of the biofilm (Fig. 5).
In case of microorganism overgrowth, their pathogenicity is regulated by transcriptional control of polymer molecule adhesion, invasion, and synthesis [32][33]. QS molecules are considered as promising targets in the development of the medicinal products that significantly reduce APEC adhesion and inhibit anti-inflammatory cytokine expression [34][35].
The results of biofilm dynamics studies will be useful for optimization of methods for microbiological monitoring of critical control points in poultry production, and can also be used for development of medicinal products and disinfectants blocking synthesis of intercellular communication molecules.
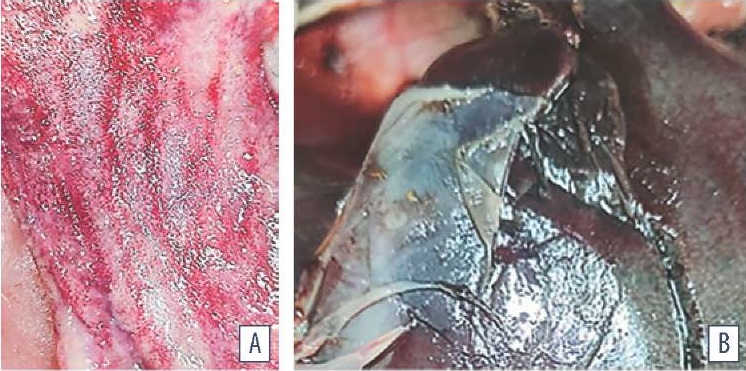
Fig. 1. Postmortem gastrointestinal lesions in poultry: A – multiple hemorrhages in intestinal mucosa; B – perihepatitis
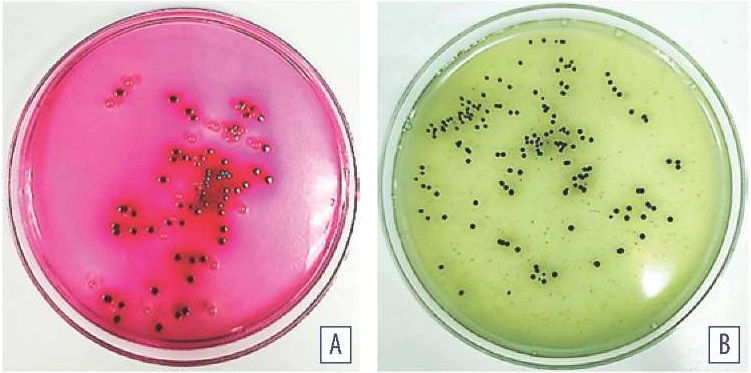
Fig. 2. Microorganism cultures isolated from chicken small intestine contents: A – Endo medium, cultivation at (37 ± 1) °С for 24 hours; B – BSA cultivation at (37 ± 1) °С for (24± 1) hours
Table
Densitometric parameters of biofilms
|
Sample cultivation time, hours |
Absolute value of optical density |
Biofilm formation intensity |
|
6 |
(0.102 ± 0.04) – (0.111 ± 0.06) |
≥ 0.1–0.2 |
|
18 |
(0.172 ± 0.07) – (0.191 ± 0.05) |
≥ 0.1–0.2 |
|
24 |
(0.246 ± 0.03) – (0.284 ± 0.08) |
≥ 0.2–0.3 |
|
48 |
(0.348 ± 0.07) – (0.526 ± 0.18) |
≥ 0.3–0.4 |
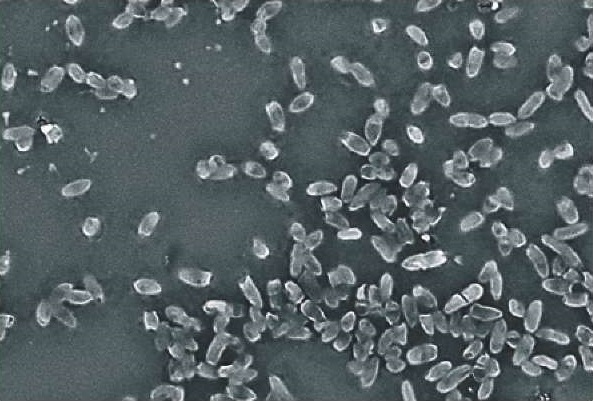
Fig. 3. E. coli biofilm morphology (MPB medium; cultivation at (37 ± 1) °C for 18 hours; Hitachi TM3030 Plus, Japan)
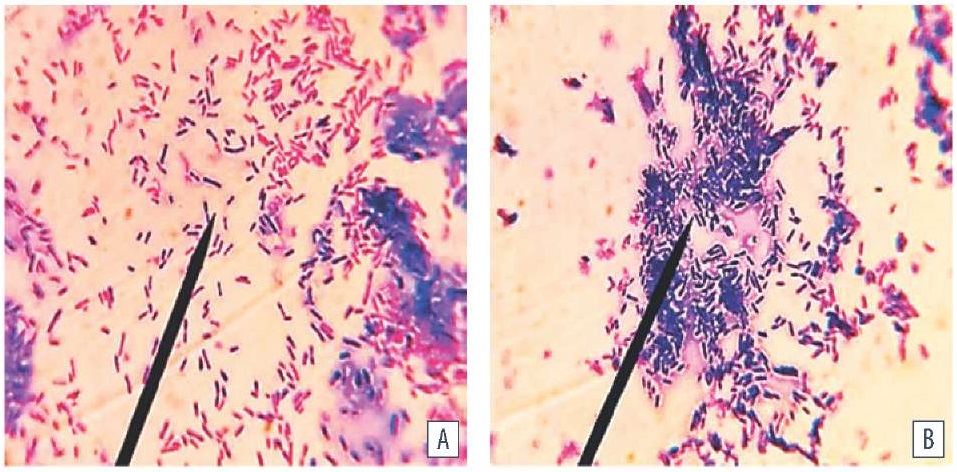
Fig. 4. E. coli biofilm morphology (MPB medium, temperature (37 ± 1) °C, cultivation period: A –18 hours, B – 24 hours; Gram staining; оc. 10×, obj. 100×, immersion, H604 Trinocular Unico, USA)
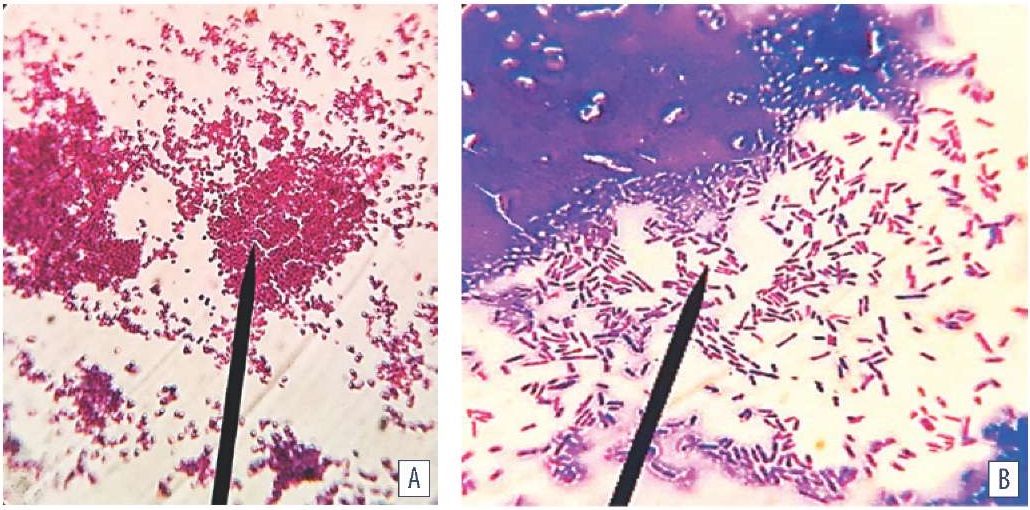
Fig. 5. Biofilm morphology: A – E. albertii; B – E. coli (MPB medium; cultivation at (37 ± 1) °C for 48 hours; Gram staining; oc. 10×, obj. 100×, immersion, H604 Trinocular Unico, USA)
CONCLUSION
E. coli, E.albertii, and P. vulgaris isolates cultivated at (37 ± 1) °C for 6, 18, 24, 48 hours under static aerobic conditions were shown to have common patterns for biofilm formation and growth. Biofilm initiation and growth is multi-stage process where motile planktonic microorganisms differentiate into an attached, structured form, with QS playing a crucial role in intercellular communication. Coaggregation of heteromorphic cells of different sizes and shapes depending on the cell cycle stage is the general pattern of heterogeneous microorganism population dynamics mediated by adhesion, intensive cell proliferation, and exocellular molecule synthesis. Bacteria characteristic for L-transformation dominated during heteromorphic population dispersion. Spheroplasts, needle-like and giant structures as well as cells capable of reverting to their original phenotypic and metabolic state were differentiated together with the cells typical of the species.
References
1. Janda J. M., Abbott S. L. The changing face of the family Enterobacteriaceae (Order: “Enterobacterales”): New members, taxonomic issues, geographic expansion, and new diseases and disease syndromes. Clinical Microbiology Reviews. 2021; 34 (2):e00174-20. https://doi.org/10.1128/cmr.00174-20
2. Mirzaei A., Nasr Esfahani B., Ghanadian M., Moghim S. Alhagi maurorum extract modulates quorum sensing genes and biofilm formation in Proteus mirabilis. Scientific Reports. 2022; 12 (1):13992. https://doi.org/10.1038/s41598-022-18362-x
3. Muchaamba F., Barmettler K., Treier A., Houf K., Stephan R. Microbiology and epidemiology of Escherichia albertii – an emerging elusive foodborne pathogen. Microorganisms. 2022; 10 (5):875. https://doi.org/10.3390/microorganisms10050875
4. Hirose S., Konishi N., Sato M., Suzumura K., Obata H., Ohtsuka K., et al. Growth and survival of Escherichia albertii in food and environmental water at various temperatures. Journal of Food Protection. 2024; 87 (4):100249. https://doi.org/10.1016/j.jfp.2024.100249
5. WHO bacterial priority pathogens list, 2024: Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Geneva: WHO; 2024. https://www.who.int/publications/i/item/9789240093461
6. Khairullah A. R., Afnani D. A., Riwu K. H. P., Widodo A., Yanestria S. M., Moses I. B., et al. Avian pathogenic Escherichia coli: epidemiology, virulence and pathogenesis, diagnosis, pathophysiology, transmission, vaccination, and control. Veterinary World. 2024; 17 (12): 2747–2762. https://doi.org/10.14202/vetworld.2024.2747-2762
7. Nawaz S., Wang Z., Zhang Y., Jia Y., Jiang W., Chen Z., et al. Avian pathogenic Escherichia coli (APEC): current insights and future challenges. Poultry Science. 2024; 103 (12):104359. https://doi.org/10.1016/j.psj.2024.104359
8. Javadov E. J., Novikova O. B., Kraskov D. A., Berezkin V. A. Bolezni ptits, vyzyvaemye uslovno-patogennoi mikrofloroi = Avian diseases caused by opportunistic microorganisms. Effectivnoe zhivotnovodstvo. 2023; (6): 8–12. https://doi.org/10.24412/cl-33489-2023-6-8-12 (in Russ.)
9. Gerasimova A. O., Novikova O. B., Savicheva A. A. Avian colibacillosis – current aspects. Veterinary Science Today. 2023; 12 (4): 284–292. https://doi.org/10.29326/2304-196X-2023-12-4-284-292
10. Kurmakaeva T. V., Kozak S. S., Baranovich E. S. On occurrence of some avian bacterial diseases and biosafety provision. Veterinary Science Today. 2024; 13 (2): 171–176. https://doi.org/10.29326/2304196X-2024-13-2-171-176
11. Makavchik S. A., Smirnova L. I., Sukhinin A. A., Kuzmin V. A. Species diversity of dominant etiologically significant bacteria circulating in industrial poultry. International Journal of Veterinary Medicine. 2022; (1): 22–26. https://doi.org/10.52419/issn2072-2419.2022.1.22 (in Russ.)
12. Tambiev T. S., Tambieva Yu. G., Duletov E. G., Fedorov V. Kh., Tazayan A. N., Fedyuk V. V., Shlychkov A. E. Antimicrobial activity of phytogenic drugs against conditionally pathogenic intestinal microflora of chickens. Actual Questions of Veterinary Biology. 2023; (2): 27–31. https://doi.org/10.24412/2074-5036-2023-2-27-31 (in Russ.)
13. Pancratov S. V., Rozhdestvenskaya T. N., Sukhinin A. A., Ruzina A. V. Poultry respiratory syndrome. Etiology. Diagnostics. Measures of control and prevention. Poultry & Chicken Products. 2021; (4): 34–36. https://elibrary.ru/tfcyys (in Russ.)
14. Isakova M. N., Sokolova O. V., Bezborodova N. A., Krivonogova A. S., Isaeva A. G., Zubareva V. D. Antimicrobial resistance in clinical Escherichia coli isolates obtained from animals. Veterinary Science Today. 2022; 11 (1): 14–19. https://doi.org/10.29326/2304-196X-2022-11-1-14-19
15. Konishcheva A. S., Leshcheva N. A., Pleshakova V. I. Pathogens microbiological spectrum in gastrointestinal pathology in animals. Bulletin KrasSAU. 2022; (2): 106–112. https://doi.org/10.36718/1819-4036-2022-2106-112 (in Russ.)
16. Pruntova O. V., Russaleyev V. S., Shadrova N. B. Current understanding of antimicrobial resistance mechanisms in bacteria (analytical review). Veterinary Science Today. 2022; 11 (1): 7–13. https://doi.org/10.29326/2304196X-2022-11-1-7-13
17. Pirozhkov M. K., Galiakbarova A. A., Pimenov N. V. The current state of the domestic market for vaccines against colibacillosis of animals. Veterinariya, Zootekhniya i Biotekhnologiya. 2022; (2): 12–20. https://doi.org/10.36871/vet.zoo.bio.202202002 (in Russ.)
18. Svetoch E. A., Eruslanov B. V., Mitsevich I. P., Khramov M. V., Pereskokova E. S., Kartsev N. N., Fursova N. K. The algorithm for development and characterization of diagnostic latex test-systems producing at the State Research Center for Applied Microbiology and Biotechnology (part 2). Bacteriology. 2023; 8 (3): 56–67. https://obolensk.org/bacteriology/archive-numbers/item/453-svetoch2023-8-3-p56-67 (in Russ.)
19. Lenchenko E., Sachivkina N., Lobaeva T., Zhabo N., Avdonina M. Bird immunobiological parameters in the dissemination of the biofilm-forming bacteria Escherichia coli. Veterinary World. 2023; 16 (5): 1052–1060. https:// doi.org/10.14202/vetworld.2023.1052-1060
20. Peng L.-Y., Yuan M., Wu Z.-M., Song K., Zhang C.-L., An Q., et al. Anti- bacterial activity of baicalin against APEC through inhibition of quorum sensing and inflammatory responses. Scientific Reports. 2019; 9 (1):4063. https://doi.org/10.1038/s41598-019-40684-6
21. Sachivkina N., Vasilieva E., Lenchenko E., Kuznetsova O., Karamyan A., Ibragimova A., et al. Reduction in pathogenicity in yeast-like fungi by farnesol in quail model. Animals. 2022; 12 (4):489. https://doi.org/10.3390/ani12040489
22. ATCC: The Global Bioresource Center. https://www.atcc.org/products/25922
23. Methodical guidelines for pathomorphological diagnosis of animal, avian, and fish diseases in veterinary laboratories: approved by the Veterinary Department of the Ministry of Agriculture of the Russian Federation on 11 September 2000, No. 13-7-2/2137. https://base.garant.ru/71878976 (in Russ.)
24. Illustrated atlas of avian diseases. Ed. B. F. Bessarabov. Moscow: Medol; 2006. 247 p. (in Russ.)
25. Volkov M. S., Irza V. N., Varkentin A. V., Rogolyov S. V., Andriyasov A. V. Results of scientific expedition to natural biotopes of the Republic of Tyva in 2019 with the purpose of infectious disease monitoring in wild bird populations. Veterinary Science Today. 2020; (2): 83–88. https://doi.org/10.29326/2304-196X-2020-2-33-83-88
26. Gromov I. N. Pathomorphological and differential diagnostics of poultry diseases affecting primarily intestines. Animal Agriculture and Vete ri nary Medicine. 2020; (2): 27–31. https://elibrary.ru/ofnxlc (in Russ.)
27. Methodical guidelines for bacteriological diagnosis of animal colibacillosis (escherichiosis): approved by the Veterinary Department of the Ministry of Agriculture of the Russian Federation on 27 July 2000 No. 13-7-2/2117. https://docs.cntd.ru/document/555906594 (in Russ.)
28. Isolation of bacteria from the animal gastrointestinal tract and identification thereof: methodical guidelines approved by the Veterinary Department of the Ministry of Agriculture of the Russian Federation on 11 May 2004, No. 13-5-02/1043. http://gost.gtsever.ru/Data2/1/4293723/4293723844.pdf (in Russ.)
29. Methodical guidelines for bacteriological diagnosis of mixed intestinal infection in young animals caused by pathogenic enterobacteria, approved by the Veterinary Department of the Ministry of Agriculture of the Russian Federation on 11 October 1999, No. 13-7-2/1759. https://base.garant.ru/71987758 (in Russ.)
30. Carter M. Q., Carychao D., Lindsey R. L. Conditional expression of flagellar motility, curli fimbriae, and biofilms in Shiga toxin-producing Escherichia albertii. Frontiers in Microbiology. 2024; 15:1456637. https://doi.org/10.3389/fmicb.2024.1456637
31. Lenchenko E., Sachivkina N., Petrukhina O., Petukhov N., Zharov A., Zhabo N., Avdonina M. Anatomical, pathological, and histological features of experimental respiratory infection of birds by biofilm-forming bacteria Staphylococcus aureus. Veterinary World. 2024; 17 (3): 612–619. https://doi.org/10.14202/vetworld.2024.612-619
32. Robé C., Blasse A., Merle R., Friese A., Roesler U., Guenther S. Low dose colonization of broiler chickens with ESBL-/AmpC-producing Escherichia coli in a seeder-bird model independent of antimicrobial selection pressure. Frontiers in Microbiology. 2019; 10:2124. https://doi.org/10.3389/fmicb.2019.02124
33. Lenchenko E., Lozovoy D., Strizhakov A., Vatnikov Yu., Byakhova V., Kulikov E., et al. Features of formation of Yersinia enterocolitica biofilms. Veterinary World. 2019; 12 (1): 136–140. https://doi.org/10.14202/vetworld.2019.136-140
34. Sivaranjani M., McCarthy M. C., Sniatynski M. K., Wu L., Dillon J. R., Rubin J. E., White A. P. Biofilm formation and antimicrobial susceptibility of E. coli associated with colibacillosis outbreaks in broiler chickens from Saskatchewan. Frontiers in Microbiology. 2022; 13:841516. https://doi.org/10.3389/fmicb.2022.841516
35. Helmy Y. A., Kathayat D., Deblais L., Srivastava V., Closs G. Jr., Tokarski R. J., et al. Evaluation of novel quorum sensing inhibitors targeting auto-inducer 2 (AI-2) for the control of avian pathogenic Escherichia coli infections in chickens. Microbiology Spectrum. 2022; 10 (3):e00286-22. https://doi.org/10.1128/spectrum.00286-22
About the Authors
E. M. LenchenkoRussian Federation
Ekaterina M. Lenchenko, Dr. Sci. (Veterinary Medicine), Professor, Department of Veterinary Medicine,
11, Volokolamskoe highway, Moscow 125080.
V. V. Ponomarev
Russian Federation
Vladislav V. Ponomarev, Postgraduate Student, Department of Veterinary Medicine,
11, Volokolamskoe highway, Moscow 125080.
N. P. Sachivkina
Russian Federation
Nadezda P. Sachivkina, Cand. Sci. (Biology), Associate Professor, Department of Veterinary Medicine, Agrarian and Technological Institute,
6, Miklukho-Maklaya str., Moscow 117198.
Review
For citations:
Lenchenko E.M., Ponomarev V.V., Sachivkina N.P. Identification of Escherichia coli, Escherichia albertii, Proteus vulgaris biofilms detected in poultry with respiratory and gastrointestinal diseases. Veterinary Science Today. 2025;14(2):186-193. https://doi.org/10.29326/2304-196X-2025-14-2-186-193



































