Scroll to:
Characteristics of the intestinal tract microbiota in calves with various forms of acute catarrhal bronchopneumonia
https://doi.org/10.29326/2304-196X-2024-13-3-275-281
Abstract
The intestinal barrier is one of the most important components that maintain gastrointestinal homeostasis, therefore changes in bacterial composition can lead to increased intestinal permeability and the development of intestinal translocation of opportunistic microorganisms, with the subsequent development or complication of various infectious diseases. A comparative description of the microbiota of the intestinal tract of calves with compensated, subcompensated and decompensated acute catarrhal bronchopneumonia of calves was carried out in the conditions of livestock farms of Vladimir and Moscow Oblasts. Calves aged 1–3 months with acute catarrhal bronchopneumonia (n = 37) were used for the study. The severity of the disease was assessed based on clinical and laboratory tests. The samples taken from clinically healthy animals (n = 8) were used as controls. It has been shown that in calves with compensated acute catarrhal bronchopneumonia, the qualitative and quantitative composition of the intestinal microbiome does not differ from clinically healthy animals. During the clinical manifestation of subcompensated and decompensated acute catarrhal bronchopneumonia in calves, a significant quantitative and qualitative shift in the microbiome occurs in the intestines, which indicates the occurrence of dysbiosis. We believe that this area is quite relevant and requires further scrupulous research.
Keywords
For citations:
Rodionova N.Yu., Kulikov E.V., Sotnikova E.D., Prozorovskiy I.E., Vatnikov Yu.A., Rudenko V.B., Rudenko P.A. Characteristics of the intestinal tract microbiota in calves with various forms of acute catarrhal bronchopneumonia. Veterinary Science Today. 2024;13(3):275-281. https://doi.org/10.29326/2304-196X-2024-13-3-275-281
INTRODUCTION
The number of cattle in livestock complexes has recently increased due to dairy farming intensification. This, in turn, creates unfavorable conditions resulting in reduced animal resistance to various adverse environmental effects, including the negative impact of opportunistic microflora associations circulating in farm biogeocenoses [1-3]. Factor infections are quite common in cattle and cause significant economic losses in livestock. The most common factor infections include obstetric and gynecological diseases in cows, as well as pneumoenteritis in calves. To prevent these cattle infections, it is necessary to follow preventive measures, including regular vaccination, maintaining hygiene in animal pens, monitoring feeding and watering, as well as regular examination and treatment of diseased animals [4-6].
Respiratory diseases which are most often diagnosed in young animals are currently widespread in livestock farms among highly productive animals. At the same time, economic losses for the industry comprise animal mortality, drop in diseased or recovered animal productivity, retarded growth and development, and costs on treatment and prevention [7]. Bronchopneumonia of calves is registered in nearly all areas of our country and it ranks second after gastrointestinal diseases among all in-farm animal pathologies, reaching 20–30% [8]. The etiological factors of nonspecific bronchopneumonia of calves are a complex of causes: high density of animal housing, decreased resistance and immunological reactivity of newborn animals, exposure to adverse environmental factors, stress, feeding imbalance, as well as conditionally pathogenic microbiota of the respiratory tract, which under these conditions can acquire pathogenic properties [9-11].
Trillions of microorganisms living in the intestinal tract are important health regulators, therefore, qualitative and quantitative disorders in microbial biotopes of the intestine can cause or complicate various infectious diseases [12-15]. Leaky gut syndrome is a condition characterized by increased intestinal permeability. Since the intestinal barrier is one of the most important structures supporting homeostasis in the gastrointestinal tract, loss of its integrity due to changes in bacterial composition, decreased levels of expression of dense compound proteins and increased concentrations of proinflammatory cytokines can lead to increased intestinal permeability with the subsequent development of gastrointestinal and non-gastrointestinal diseases. The translocation of microorganisms and their toxic metabolites outside the biotope being the gastrointestinal tract, is one of the consequences of the leaky gut syndrome [16-18].
Translocation of the intestinal microbiota is a process where microorganisms from the intestinal tract penetrate through its wall into the bloodstream and distribute in the body. This may play a significant role in the development of infectious diseases of various etiology, including respiratory ones. Thus, pathogenic microorganisms entering the bloodstream by intestinal translocation can cause a systemic inflammatory response in the body, thus deteriorating the course of infection. Also, bacteria penetrating through the intestinal wall may cause metastatic infections in various organs and tissues, leading to complications and a more severe course of the disease. In addition, intestinal translocation of microorganisms can facilitate bacteremia, which might result in sepsis and shock. The entry of infectious agents from the intestinal tract into the bloodstream may also cause recurrence of infection after completion of antibiotic therapy [19-22].
In general, intestinal translocation of microorganisms plays an important role in the development and course of various infectious diseases, therefore, control of this process may be a key aspect in the treatment of such conditions. In this regard, the study of the role of intestinal translocation of microorganisms in acute catarrhal bronchopneumonia of calves of varying severity is an urgent issue requiring timely and competent solutions.
The novelty of the paper consists in the fact that it is for the first time that the condition of the rectal biotope in calves with acute catarrhal bronchopneumonia of varying severity was studied. It has been shown that, depending on the severity of the infectious process, significant qualitative and quantitative shifts occur in the setting of intestinal dysbiosis.
The aim of the work is to conduct a comparative analysis of the intestinal microbiota in calves with compensated, subcompensated and decompensated acute catarrhal bronchopneumonia on the livestock farms in the Vladimir and Moscow Oblasts.
MATERIALS AND METHODS
The research was supported by the grants of the Russian Science Foundation (project No. 24-26-00091, https://rscf.ru/project/24-26-00091) and conducted on the basis of livestock farms in the Vladimir and Moscow Oblasts with a total cattle population of 3,680 animals, including 1,690 cows.
Bacteriological tests were performed in the VETTEST veterinary laboratory (Moscow).
The study was aimed at 1–3 months-old calves with acute catarrhal bronchopneumonia (n = 37). The diagnosis was established based on comprehensive data, taking into account the medical history, clinical examination and microbiological tests. The animals that received medical treatment within 14 days prior to sampling were removed from the experiment. The severity of acute catarrhal bronchopneumonia of calves (1st degree – compensated; 2nd degree – subcompensated; 3rd degree – decompensated) was assessed on the basis of clinical and laboratory tests. The animals were divided into three groups based on the severity of bronchopneumonia: calves with the mild degree (compensated, n = 12), moderate degree (subcompensated, n = 14) and severe degree (decompensated, n = 11) of the disease severity. The samples collected from clinically healthy animals were used as controls (n = 8).
Fecal samples were collected from experimental calves in the morning hours and placed into sterile test tubes. For microbiological tests, the pathological samples were inoculated onto nutrient media using a Pasteur pipette. Sabouraud glucose agar was used for yeast-like fungi; peptone-salt medium, yolk-salt agar and meat-peptone agar (MPA) were used for staphylococci; Endo agar, Ploskirev medium, King medium and bismuth-sulphite agar – for enterobacteria; Blaurocca medium – for bifidobacteria; skimmed milk and MRS agar – for lactobacilli. The inoculations were then incubated in a thermostat at 37–38 °C for 24 hours, and if no growth was observed, the dishes were kept longer for up to 3 days. After testing the cultural and morphological properties, all species colonies were separately inoculated into test tubes and incubated at 37–38 °C for 24 hours. The resulting pure bacterial cultures were tested for mobility in crushed droplet preparations using phase contrast microscopy in a darkened field of view and subjected to identification.
For quantitative bacteriological test, 1.0 g of feces were collected and added into sterile tubes with sterile sodium chloride saline solution (9.0 cm3). The contents of the first tube, considered as 10–1 dilution, was used for preparing further ten-fold dilutions up to 10–10. Then, 0.1 cm3 of the resulting mixture from each tube was inoculated into Petri dishes onto solid nutrient media (Endo, MPA, Sabouraud, Blaurocca, MRS, PSL, King, Ressel, bismuth-sulphite agar, yolk-salt agar).
The number of microorganisms (C) in 1.0 cm3 of feces collected from calves with acute catarrhal bronchopneumonia was calculated using the formula below and expressed in logarithms with a base of 10:
C = (N/V) × K,
where N is the mean number of colonies in one bacteriological dish; V is the volume of suspension applied during inoculation onto agar (cm3); K is the multiplicity of dilution.
The morphology of bacteria was tested in smears stained according to Gram and Romanovsky – Giemsa. Further identification based on biochemical properties was carried out in accordance with Bergey’s Manual of Determinative Bacteriology1.
The obtained test results were processed statistically and presented in the tables. All calculations were performed using the STATISTICA 7.0 program (StatSoft, USA), while the normality of the distribution was preliminarily estimated using Shapiro – Wilk tests. In case of a normal distribution of quantitative variables, the Student’s independent samples t-test was used to compare the two groups. The arithmetic mean (Mean), standard error (SE) and standard deviation (SD) were calculated. The reliability of the analyte difference between the indicators of the control and experimental groups was calculated using the Mann – Whitney method2.
RESULTS AND DISCUSSION
During the epizootological examination, 37 calves (1–3 months of age) with acute catarrhal bronchopneumonia were identified. Based on clinical study results 12 animals were classified as having a mild, compensated disease stage (slight depression, subfebrile body temperature, shallow hard breathing, serous nasal discharge), 14 calves had a medium, subcompensated disease stage (depression, fever, increased pulse and respiration, dry wheezing and cough, abundant serous catarrhal discharge), and 11 calves had a severe, decompensated disease stage (pronounced depression and exhaustion, inappetence, decreased reaction to external stimuli, fever, increased pulse and respiration, painful dry cough, wheezing, foci of dulling percussion sound, abundant serous-catarrhal exudate of greenish color).
Microbiological studies of bronchoalveolar lavage samples collected from diseased animals have shown that the occurrence of bronchopneumonia in calves is due to a fairly wide range of conditionally pathogenic microflora. Thus, 115 bacteria of thirteen species classified into nine genera were isolated from the bronchial samples. At the same time, the following strains were isolated more often from bronchoalveolar lavage samples: Staphylococcus aureus – 18 (15.6%) cultures, Mannheimia haemolytica – 18 (15.6%) strains, Escherichia coli – 15 (13.1%) isolates, Pasteurella multocida – 11 (9.6%) cultures and Klebsiella pneumonia – 11 (9.6%) strains. Staphylococcus intermedius and Proteus mirabilis were isolated much less frequently – three (2.6%) cases per each species, respectively.
The quantity of microorganisms (lg) in 1 g of feces from calves with compensated, subcompensated and decompensated acute catarrhal bronchopneumonia is shown in the Table.
The analysis of the results showed that the qualitative and quantitative composition of the intestinal microbiome in calves with compensated acute catarrhal bronchopneumonia does not differ from that in clinically healthy animals.
The presented data demonstrate that significant quantitative and qualitative changes occur in the intestinal biotope of animals with a clinical manifestation of moderate severity of acute catarrhal bronchopneumonia, which indicate the development of dysbiosis. The fecal samples from calves with subcompensated bronchopneumonia demonstrated a reliable increase in Enterobacter spp. by 1.26 times (p < 0.05), Citrobacter spp. by 1.35 times (p < 0.05), Klebsiella spp. by 1.90 times (p < 0.01), Proteus spp. by 1.66 times (p < 0.01), Pseudomonas spp. by 2.58 times (p < 0.001), Clostridium spp. by 2.06 times (p < 0.001) and Candida spp. by 2.12 times (p < 0.01) when compared with the indicators for the control group animals. This was observed against the background of a highly reliable (p < 0.001) decrease in representatives of the genera Lactobacillus and Bifidobacterium from (10.42 ± 0.72) to (8.59 ± 0.76) lg and from (10.94 ± 0.73) to (9.06 ± 0.62) lg, by 17.56 and 17.18%, respectively, when compared with the indicators for clinically healthy calves.
The results shown in the Table also indicate that significant quantitative and qualitative dysbiotic shifts in the microbiota of the intestinal tract were observed in calves with the most severe decompensated degree of acute catarrhal bronchopneumonia. Thus, the testing of fecal samples from diseased animals showed a significant increase in the amount of representatives of the following genera: Escherichia by 1.21 times (p < 0.001), Enterobacter by 1.63 times (p < 0.001), Citrobacter by 1.83 times (p < 0.001), Klebsiella by 3.08 times (p < 0.001), Proteus by 1.90 times (p < 0.001), Pseudomonas by 3.57 times (p < 0.001), Staphylococcus by 1.24 times (p < 0.05), Streptococcus by 1.38 times (p < 0.01), Bacillus by 1.47 times (p < 0.01), Clostridium by 3.34 times (p < 0.001) and yeast fungi (genus Candida) by 2.82 times (p < 0.001), when compared with the indicators for the control group animals. The presented quantitative differences were recorded against the background of a highly reliable decrease in the amount of the following species in fecal samples of experimental animals: Lactobacillus spp. (p < 0.001) and Bifidobacterium spp. (p < 0.001) from (10.42 ± 0.72) to (6.51 ± 1.08) lg and from (10.94 ± 0.73) to (7.36 ± 0.81) lg, by 37.5 and 32.7%, respectively, as compared with the control group. That was the first time that we obtained these data.
The qualitative parameters of the intestinal microflora in calves with acute catarrhal bronchopneumonia of compensated, subcompensated and decompensated severity are shown in the Figure.
As we can see, the representatives of the genera Klebsiella, Pseudomona and Clostridium were not detected in the most mild, compensated forms of the disease, however, were isolated in the more severe course of the disease – subcompensated and decompensated degrees of severity. It can be assumed that the severity of bronchopneumonia may be associated with intestinal dysbiosis and the phenomenon of intestinal translocation of microorganisms from a natural, evolutionarily developed biotope to the focus of destruction – the lungs. Therefore, further studies should be aimed at determining the identity of the intestinal microflora with microorganisms isolated from lung tissue in the setting of bronchopneumonia.
Thus, the new criteria for assessing the severity of acute catarrhal bronchopneumonia in calves have been suggested. To this extent, when subcompensated and decompensated acute catarrhal bronchopneumonia is clinically manifested in calves, significant quantitative and qualitative microbiome disorders occur in the intestine, indicating dysbiosis. The development of intestinal dysbiosis may serve as a kind of trigger for the formation and progression of pathologies of the respiratory tract. The data obtained comply with the study results on intestinal microbiome in inflammatory processes of various localization [20, 23]. This confirms the important role of the intestinal microbiota and intestinal permeability (normal and increased) in the manifestation of many infectious diseases. However, the currently available data are selective and insufficient, their assessment is a matter of debate, and their clinical significance requires additional studies. In this regard, we believe that the study of intestinal microflora in animals during inflammatory and infectious processes requires further scrupulous investigation.
Table
Composition and quantity (lg) of opportunistic microflora in 1 g of calf feces in various forms of acute catarrhal bronchopneumonia (М ± m)
|
Microorganism genus |
Control (n = ٨) |
Mild degree (n = 12) |
Medium degree (n = 14) |
Severe degree (n = ١١) |
|
Escherichia |
7.07 ± 0.96 |
7.40 ± 0.77 |
7.69 ± 0.79 |
8.55 ± 0.61*** |
|
Enterobacter |
2.65 ± 0.70 |
2.72 ± 0.77 |
3.35 ± 0.50* |
4.31 ± 0.82*** |
|
Citrobacter |
2.26 ± 0.75 |
2.34 ± 0.78 |
3.05 ± 0.84* |
4.14 ± 0.70*** |
|
Klebsiella |
0 |
0 |
1.90 ± 1.43** |
3.08 ± 1.02*** |
|
Proteus |
1.79 ± 0.51 |
2.01 ± 0.69 |
2.97 ± 0.85** |
3.40 ± 0.76*** |
|
Pseudomonas |
0 |
0 |
2.58 ± 0.72*** |
3.57 ± 0.78*** |
|
Staphylococcus |
3.61 ± 0.78 |
3.50 ± 0.71 |
3.93 ± 0.75 |
4.47 ± 0.76* |
|
Streptococcus |
3.59 ± 0.75 |
3.26 ± 0.91 |
4.17 ± 0.65 |
4.95 ± 0.78** |
|
Bacillus |
2.74 ± 0.89 |
2.65 ± 0.85 |
3.13 ± 0.49 |
4.02 ± 0.97** |
|
Clostridium |
0 |
0 |
2.06 ± 1.13*** |
3.34 ± 0.67*** |
|
Lactobacillus |
10.42 ± 0.72 |
10.27 ± 0.67 |
8.59 ± 0.76*** |
6.51 ± 1.08*** |
|
Bifidobacterium |
10.94 ± 0.73 |
10.72 ± 0.84 |
9.06 ± 0.62*** |
7.36 ± 0.81*** |
|
Candida |
1.29 ± 1.48 |
1.28 ± 1.27 |
2.74 ± 0.77** |
3.64 ± 0.88*** |
|
* р < 0.05; ** р < 0.01; *** р < 0.001 as compared with the control group. |
||||
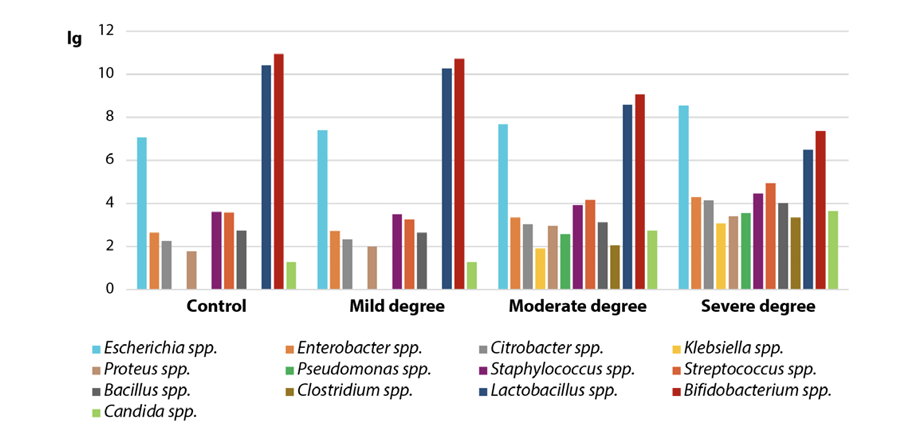
Fig. Qualitative changes in the intestinal microflora in calves with acute catarrhal bronchopneumonia of varying severity
CONCLUSION
The paper gives a detailed description of the intestinal tract microbiota in calves with acute catarrhal bronchopneumonia with varying degrees of severity of the infectious process. It has been shown that the qualitative and quantitative composition of the intestinal microbiome in calves with compensated acute catarrhal bronchopneumonia does not differ from clinically healthy animals. It has been established that significant quantitative and qualitative shifts occur in the intestinal biotope of animals in case of subcompensated acute catarrhal bronchopneumonia, indicating the dysbiosis. Thus, the fecal samples showed a reliable increase in the number of the following genera populations: Enterobacter, Citrobacter, Klebsiella, Proteus, Pseudomonas, Clostridium and Candida, against the background of a highly reliable decrease in the genera Lactobacillus and Bifidobacterium by 17.56 and 17.18%, respectively, when compared with the indicators of clinically healthy calves. Even more significant quantitative and qualitative dysbiotic shifts in the intestinal tract microbiota were observed in calves with the most severe decompensated form of acute catarrhal bronchopneumonia. Thus, the fecal samples from diseased animals showed a reliable increase in the number of the following genera populations: Escherichia, Enterobacter, Citrobacter, Klebsiella, Proteus, Pseudomonas, Staphylococcus, Streptococcus, Bacillus, Clostridium and yeast fungi (genus Candida) when compared with the indicators of animals in the control group. This was revealed against the background of a highly reliable decrease in fecal samples of experimental animals of the Lactobacillus and Bifidobacterium genera populations by 37.5 and 32.7%, respectively, as compared with the control group. It should be noted that in case of subcompensated and decompensated acute catarrhal bronchopneumonia, the representatives of the genera Klebsiella, Pseudomonas and Clostridium were found in samples of feces of calves, unlike healthy animals and animals with a mild degree of pathology.
1. Holt J. G., Krieg N. R., Sneath P. H. A., Staley J. T., Williams S. T. Bergey’s Manual of Determinative Bacteriology. 9th Baltimore: Williams & Wilkins; 1994. 787 p.
2. Rebrova O. Yu. Statistical analysis of medical data. Application of the STATISTICA software package. Moscow: Media Sphere; 2002. 312 p. (in Russ.)
References
1. Wang Y., Zhang P., Wu J., Chen S., Jin Y., Long J., et al. Transmission of livestock-associated methicillin-resistant Staphylococcus aureus between animals, environment, and humansin the farm. Environmental Science and Pollution Research. 2023; 30 (37): 86521–86539. https://doi.org/10.1007/s11356-023-28532-7
2. Van Boeckel T. P., Brower C., Gilbert M., Grenfell B. T., Levin S. A., RobinsonT. P., et al. Globaltrendsin antimicrobial use in food animals. Proceedings of the National Academy of Sciences. 2015; 112 (18): 5649–5654. https://doi.org/10.1073/pnas.1503141112
3. Alarcon P., Strang C. L., Chang Y. M., Tak M. Economic evaluation of antimicrobial usage surveillance in livestock. Scientific & Technical Review. 2023; 42: 42–51. https://doi.org/10.20506/rst.42.3347
4. Rudenko A., Glamazdin I., Lutsay V., Sysoeva N., Tresnitskiy S., Rudenko P. Parasitocenoses in cattle and their circulation in small farms. E3S Web of Conferences. 2022; 363:03029. https://doi.org/10.1051/e3sconf/202236303029
5. Peng Z., Hu Z., Li Z., Zhang X., Jia C., LiT., et al. Antimicrobialresistance and population genomics of multidrug-resistant Escherichia coli in pig farms in mainland China. Nature Communications. 2022; 13:1116. https://doi.org/10.1038/s41467-022-28750-6
6. Patterson L., Navarro-Gonzalez N., Jay-Russell M. T., Aminabadi P., Pires A. F. A. Risk factors of Shiga toxin-producing Escherichia coli in livestock raised on diversified small-scale farmsin California. Epidemiology and Infection. 2022; 150:e125. https://doi.org/10.1017/S0950268822001005
7. Buczinski S., Pardon B. Bovine respiratory disease diagnosis: What progress has been made in clinical diagnosis? Veterinary Clinics of North America: Food Animal Practice. 2020; 36 (2): 399–423. https://doi.org/10.1016/j.cvfa.2020.03.004
8. Sergeyeva N. N., Dedkova A. I. The efficacy of different treatment schemesfor bronchopneumonia of calves. Bulletinof AgrarianScience. 2021; (5): 64–68. https://doi.org/10.17238/issn2587-666X.2021.5.64 (in Russ.)
9. Gorpinchenko E. A., Lifentsova M. N., Zaiko K. S., Ratnikov A. R. Pharmacoprophylaxis of calves non-specific bronchopneumonia by using aerosols. Russian Journal of Veterinary Pathology. 2021; (3): 24–33. https://doi.org/10.25690/VETPAT.2021.80.88.001 (in Russ.)
10. Kalaeva E., Kalaev V., Chernitskiy A., Alhamed M., Safonov V. Incidence risk of bronchopneumonia in newborn calves associated with intrauterine diselementosis. Veterinary World. 2020; 13 (5): 987–995. https://doi.org/10.14202/vetworld.2020.987-995
11. Kuchmenko T., Shuba A., Umarkhanov R., Chernitskiy A. Portable electronic nose for analyzing the smell of nasalsecretionsin calves: Toward noninvasive diagnosis of infectious bronchopneumonia. Veterinary Sciences. 2021; 8 (5):74. https://doi.org/10.3390/vetsci8050074
12. Rudenko P. A. Modern approachesto the fight againstinflammatory processes in small animals. Russian Veterinary Journal. Small Domestic and Wild Animals. 2016; (3): 26–29. https://elibrary.ru/waifrh (in Russ.)
13. Hsu C. L., Schnabl B. The gut-liver axis and gut microbiota in health and liver disease. Nature Reviews Microbiology. 2023; 21 (11): 719–733. https://doi.org/10.1038/s41579-023-00904-3
14. Zhang K., Wang N., Lu L., Ma X. Fermentation and metabolism of dietary protein by intestinal microorganisms. Current Protein & Peptide Science. 2020; 21 (8): 807–811. https://doi.org/10.2174/1389203721666200212095902
15. Andrade M. E. R., Araújo R. S., de Barros P. A. V., Soares A. D. N., Abrantes F. A., de Vasconcelos Generoso S. V., et al. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clinical Nutrition. 2015; 34 (6): 1080–1087. https://doi.org/10.1016/j.clnu.2015.01.012
16. Twardowska A., Makaro A., Binienda A., Fichna J., Salaga M. Preventing bacterial translocation in patients with leaky gut syndrome: nutrition and pharmacological treatment options. InternationalJournal of Molecular Sciences. 2022; 23 (6):3204. https://doi.org/10.3390/ijms23063204
17. Bischoff S. C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.-D., Serino M., et al. Intestinal permeability – a new targetfor disease prevention and therapy. BMC Gastroenterology. 2014; 14:189. https://doi.org/10.1186/s12876-014-0189-7
18. Stanley D., Mason L. J., Mackin K. E., Srikhanta Y. N., Lyras D., Prakash M. D., et al. Translocation and dissemination of commensal bacteria in post-stroke infection. NatureMedicine. 2016; 22 (11): 1277–1284. https://doi.org/10.1038/nm.4194
19. Ardatskaya M. D., Shevtsov V. V., Zhakot A. N., Fedankov I. N., Mitrokhin S. D., Mironov A. Yu., Ponomareva E. V. Microflora metabolites of different habitats in bronchopulmonary diseases. Experimental and Clinical Gastroenterology. 2014; (3): 46–54. https://elibrary.ru/szuuwt (in Russ.)
20. Rudenko P. A. The role of intestinal dysbacteriosis in the mechanisms of formation and progress of surgical infection in cats. Scientific Life. 2018; (1): 84–98. https://elibrary.ru/ystjek (in Russ.)
21. Orlando G., Pisani F., Mastrantonio P., Bonanni L., Di Cocco P., D’Angelo M., et al. Eubacterium plautii infection in a kidney transplant recipient: a noteworthy case of pleural effusion and fever. Clinical Transplantation. 2008; 22 (4): 520–524. https://doi.org/10.1111/j.1399-0012.2008.00805.x
22. Pernomian L., Duarte-Silva M., de Barros Cardoso C. R. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: Insights from an immune and bacteria sensor receptor. Clinical Reviews in Allergy & Immunology. 2020; 59 (3): 382–390. https://doi.org/10.1007/s12016-020-08789-3
23. Zysset-BurriD. C., Morandi S., HerzogE. L., Berger L. E., ZinkernagelM. S. The role of the gut microbiome in eye diseases. Progressin Retinal and Eye Research. 2023; 92:101117. https://doi.org/10.1016/j.preteyeres.2022.101117
About the Authors
N. Yu. RodionovaRussian Federation
Natalia Yu. Rodionova - Assistant, Department of Veterinary Medicine, Agrarian and Technological Institute
6 Miklukho-Maklaya str., Moscow 117198
E. V. Kulikov
Russian Federation
Evgeny V. Kulikov - Cand. Sci. (Biology), Associate Professor, Department of Veterinary Medicine, Agrarian and Technological Institute
6 Miklukho-Maklaya str., Moscow 117198
E. D. Sotnikova
Russian Federation
Elena D. Sotnikova -Cand. Sci. (Biology), Associate Professor, Department of Veterinary Medicine, Agrarian and Technological Institute
6 Miklukho-Maklaya str., Moscow 117198
I. E. Prozorovskiy
Russian Federation
Ivan E. Prozorovskiy - Assistant, Department of Veterinary Medicine, Agrarian and Technological Institute
6 Miklukho-Maklaya str., Moscow 117198
Yu. A. Vatnikov
Russian Federation
Yury A. Vatnikov - Dr. Sci. (Veterinary Medicine), Professor, Director of the Department of Veterinary Medicine, Agrarian and Technological Institute
6 Miklukho-Maklaya str., Moscow 117198
V. B. Rudenko
Russian Federation
Victoria B. Rudenko - Junior Researcher, Laboratory of Biological Testing
6 Prospekt Nauki, Pushchino 142290, Moscow Oblast
P. A. Rudenko
Russian Federation
Pavel A. Rudenko - Dr. Sci. (Veterinary Medicine), Professor, Department of Veterinary Medicine, Agrarian and Technological Institute
6 Miklukho-Maklaya str., Moscow 117198
Review
For citations:
Rodionova N.Yu., Kulikov E.V., Sotnikova E.D., Prozorovskiy I.E., Vatnikov Yu.A., Rudenko V.B., Rudenko P.A. Characteristics of the intestinal tract microbiota in calves with various forms of acute catarrhal bronchopneumonia. Veterinary Science Today. 2024;13(3):275-281. https://doi.org/10.29326/2304-196X-2024-13-3-275-281
JATS XML



































