Scroll to:
Studying immunotherapeutic properties of the conjugate based on BCG antigens with betulonic acid in guinea pigs infected with Mycobacterium scrofulaceum
https://doi.org/10.29326/2304-196X-2024-13-2-183-188
Abstract
The paper reports on the research into the immunotherapeutic properties of a conjugate based on BCG antigens with betulonic acid after experimental infection of guinea pigs with Mycobacterium scrofulaceum culture, belonging to nontuberculosis mycobacteria type II according to the Runyon classification. Fifteen guinea pigs were used for the experimental purposes, divided into 3 groups. Mycobacterium scrofulaceum was subcutaneously injected into animals of Groups 1 and 2 (n = 10) at a dose of 5 mg. Fourteen days later, a conjugate based on BCG antigens with betulonic acid was subcutaneously injected into animals of Group 2 (n = 5) at a dose of 500 µg/mL of protein. Five intact animals were used as controls. During the experiment, neutrophil bactericidal activity was assessed, and histopathological examination of inguinal lymph nodes was done. The experiment showed that the inoculation of Mycobacterium scrofulaceum into guinea pigs activates cationic proteins and neutrophil myeloperoxidase, and on experiment day 42 (preceded by mycobacteria withdrawal from the body) their concentration reduced to the level of the control group. The vaccine administration induced a more active intracellular phagocyte metabolism during the entire observation period, which resulted in the elimination of nontuberculosis mycobacteria in animals as early as day 7 after treatment with the conjugate. The elimination was confirmed by the absence of mycobacterial antigen in blood smears tested in indirect immunofluorescence, as well as by histopathological changes in inguinal lymph nodes demonstrated as a reduction of germinal centers within lymphoid follicles.
Keywords
For citations:
Koshkin I.N., Vlasenko V.S., Dengis N.A. Studying immunotherapeutic properties of the conjugate based on BCG antigens with betulonic acid in guinea pigs infected with Mycobacterium scrofulaceum. Veterinary Science Today. 2024;13(2):183-188. https://doi.org/10.29326/2304-196X-2024-13-2-183-188
INTRODUCTION
Out of more than 190 currently known species of Mycobacterium genus a significant number belongs to non-tuberculous mycobacteria and over 60 species are pathogenic to animals and humans [1][2].
Non-tuberculous mycobacteria may be found ubiquitously in the environment and they pose a serious problem for in vivo and postmortem diagnosis of bovine tuberculosis as they cause false positive response to administration of tuberculin due to antigenic determinants in the allergen, which are common to non-tuberculous and pathogenic mycobacteria. In addition, visible and microscopic changes induced by non-tuberculous mycobacteria are in some cases difficult to distinguish from lesions caused by Mycobacterium tuberculosis and Mycobacterium bovis [2][3][4][5][6].
Owing to a drop in bovine tuberculosis transmission and strong diagnostic measures taken to detect residual infection in the territories where disease control programs are in place, there has been an increase in mycobacterioses caused by non-tuberculous mycobacteria [7][8][9][10]. Despite the growing interest, little data has been published so far on non-tuberculous mycobacterial infections, and the available literature is mainly focused on the Mycobacterium avium complex and its subspecies [11][12][13][14][15].
To solve the problem of non-specific reactions induced by non-tuberculous mycobacteria, specific immunoprophylactic or immunotherapeutic tools may be an extra option to complement lifetime differential tests (simultaneous, palpebral tests, etc.). Several recent studies suggest that cross-reactive response to non-tuberculous mycobacteria [16][17][18][19] is induced by BCG vaccination, as well as by immunization with areactogenic conjugates based on protective antigens, isolated from the BCG vaccine, with polyions [20]. Conversely, some scientists claim that previous contacts with non-tuberculous mycobacteria may have an antagonistic effect, reducing vaccination effectiveness; however, this concern is only about live BCG vaccine and did not affect protective properties of inactivated subunit tuberculous vaccines [21][22][23][24].
From our perspective, conjugates based on BCG antigens with betulin and its derivatives (betulonic and betulinic acids) may look promising in this regard. In particular, molecular docking has shown that betulonic acid in most cases exhibits the highest inhibitory activity against protein targets that are structural parts of Mycobacterium tuberculosis and/or Mycobacterium bovis [25].
In connection with the above, the purpose of this work is to study the immunotherapeutic efficacy of an experimental conjugate based on BCG antigens with betulonic acid.
MATERIALS AND METHODS
The experiment was conducted in Agouti guinea pigs in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes as of 18 March 1986, and was approved by the local independent ethical committee of the organization for the care and use of laboratory animals. Experimental animals were grouped based on common characteristics (weight – 400–500 g, age – 4–5 months).
From 14- to 21-day scotochromogenic mycobacteria Mycobacterium scrofulaceum (Runyon II: Scotochromogens) was used to infect experimental animals. It was administered subcutaneously into the left groin, at a dose of 5 mg/mL. Mycobacterium cultures were administered to 10 animals, further divided into 2 groups: group 1 – infected with Mycobacterium scrofulaceum (n = 5); group 2 – infected with Mycobacterium scrofulaceum and on day 14 after the administration, they were treated with conjugate of BCG antigen with betulonic acid (n = 5). The other five intact guinea pigs were used as controls.
The experimental conjugate of BCG antigenic complexes with betulonic acid was designed in accordance with the author’s development. The preparation was administered subcutaneously to animals at a dose of 500 µg/mL of protein. Betulonic acid was synthesized at the Department of Organic and Environmental Chemistry of the Institute of Chemistry of the University of Tyumen and was kindly provided for research by Professor, Dr. Sci. (Chemistry) I. V. Kulakov.
Mycobacterial antigen in blood samples was detected using indirect immunofluorescence in accordance with the methodological recommendations of N. N. Novikova et al. [26]. Myeloperoxidase activity and number of neutrophil cationic proteins were measured using benzidine test and bromophenol blue test with phagocyte distributed depending on the number of cytoplasmic granules (1st, 2nd and 3rd degrees), followed by calculation of average cytochemical coefficients (ACC) using standard methods.
Before the start of the experiment and on day 21 post infection, allergy tests were performed using intradermal administration of purified tuberculin for mammals. Blood was sampled for serological tests on day 21 and 42 after administration of scotochromogenic mycobacteria; and on days 14, 28 and 42 to assess functional status of neutrophils.
The laboratory animals were euthanized under ether anesthesia followed by total exsanguination on day 45 after the beginning of the experiment. For histological tests pieces of inguinal lymph nodes were taken (from regional lymph nodes, i.e. the closest ones to the site of mycobacteria inoculation, as well as from the lymph nodes on the opposite side). The sampled pieces were placed into cassettes and submerge in 10% neutral buffered formalin, and then the tissue was paraffin-embedded using MICROM EC 350 (Thermo Fisher Scientific Inc., USA). Rotary Microtome HM 340E (produced by Thermo Fisher Scientific Inc., USA) was used to cut sample sections, ranging between 5 and 7 µm. Histological tissue preparations were stained with hematoxylin and eosin, and then examined microscopically.
Standard methods of variational statistics were used, such as calculation of arithmetic means (M) and calculation of errors of arithmetic means (m), to process the obtained data. Student’s t-test was used to assess significance of differences (p) between the two mean values of Mx and My. The differences in the results were considered statistically significant at a significance level of p ≤ 0.05.
RESULTS AND DISCUSSION
Inoculation of Mycobacterium scrofulaceum to guinea pigs enhanced oxygen-independent mechanisms of neutrophils, as evidenced by a 1.60 and 1.74-fold increase in phagocytes with a large number of cytoplasmic granules (3rd degree) containing cationic proteins in group 1 and 2, respectively (p < 0.01), as compared to the control group. Following these changes, average cytochemical coefficients also increased by a factor of 1.65 (Table 1).
Table 1
Level of neutrophil cationic proteins in animals
at different moments post inoculation of Mycobacterium scrofulaceum, M ± m
|
Cytochemical parameters |
Group of animals |
||
|
Control |
Experimental group 1 |
Experimental group 2 |
|
|
Day 14 after inoculation of Mycobacterium |
|||
|
1st degree, % |
5.00 ± 0.58 |
11.33 ± 3.33 |
10.00 ± 3.05 |
|
2nd degree, % |
9.66 ± 1.67 |
16.66 ± 2.40 |
10.00 ± 1.15 |
|
3rd degree, % |
33.00 ± 1.15 |
52.66 ± 5.78* |
57.33 ± 4.37** |
|
Average cytochemical coefficient, conditional units |
1.23 ± 0.02 |
2.03 ± 0.11** |
2.02 ± 0.12** |
|
Day 28 after inoculation of Mycobacterium (day 14 after administration of the preparation) |
|||
|
1st degree, % |
3.33 ± 0.67 |
8.33 ± 2.85 |
3.66 ± 0.88 |
|
2nd degree, % |
14.00 ± 0.58 |
12.66 ± 2.40 |
9.66 ± 0.33** |
|
3rd degree, % |
29.33 ± 2.18 |
45.33 ± 1.33** |
57.00 ± 4.04** |
|
Average cytochemical coefficient, conditional units |
1.19 ± 0.06 |
1.70 ± 0.05** |
1.94 ± 0.11** |
|
Day 42 after inoculation of Mycobacterium (day 28 after administration of the preparation) |
|||
|
1st degree, % |
5.33 ± 2.33 |
5.00 ± 0.58 |
2.66 ± 1.76 |
|
2nd degree, % |
11.00 ± 0.58 |
11.66 ± 0.88 |
7.00 ± 1.73 |
|
3rd degree, % |
30.00 ± 4.58 |
33.33 ± 2.73 |
71.66 ± 2.03*** |
|
Average cytochemical coefficient, conditional units |
1.17 ± 0.12 |
1.28 ± 0.08 |
2.31 ± 0.08** |
*p < 0.05; **p < 0.01; ***p < 0.001.
Delayed-type hypersensitivity response to a tuberculin test conducted on day 21 post infection of guinea pigs was observed only in 60% of animals who had not received experimental preparation (group 1). Nevertheless, mycobacterial antigen was detected in all animals of this group using indirect immunofluorescence. Mean induration size in the reactors was 4.33 ± 0.33 mm.
On day 28 following sensitization of guinea pigs with non-tuberculous mycobacteria type II (according to the Runyon classification) the same trend persisted, i.e. a significant increase in concentration of neutrophil cationic proteins in the experimental groups compared to the control group. The activity of neutrophil antimicrobial peptides was higher in the group that had been treated with the experimental preparation on day 14 after inoculation of scotochromogenic mycobacteria (group 2), and was at the same level that had been observed in the test two weeks before. In contrast, the metabolic processes in group 1 were less intensive compared to the previous testing.
After another 14 days, concentration of cationic proteins in guinea pigs of group 1 dropped to the control levels. Thus, the average cytochemical coefficient within the group was 1.28 ± 0.08 c. u., and 1.17 ± 0.12 c. u. in the control. In contrast, neutrophil oxygen-dependent metabolism in the animals immunized with the experimental conjugate was more active due to an increase in the number of highly active phagocytes by 2.39 times (p < 0.001), thus, leading to a 1.97-fold increase in the average cytochemical coefficient (p < 0.01).
The administration of Mycobacterium scrofulaceum to guinea pigs also stimulated neutrophil oxygen-dependent metabolism (Table 2). Thus, the level of the average cytochemical coefficient of myeloperoxidase increased with a high degree of confidence (p < 0.01) by 1.79 and 1.82 times in both experimental groups, respectively, due to a 2-fold increase in the number of highly active phagocytes as compared to the control group.
Table 2
Enzyme activity of neutrophil myeloperoxidase in animals
at different moments post inoculation of Mycobacterium scrofulaceum, M ± m
|
Cytochemical parameters |
Group of animals |
||
|
Control |
Experimental group 1 |
Experimental group 2 |
|
|
Day 14 after inoculation of Mycobacterium |
|||
|
1st degree, % |
9.33 ± 0.67 |
9.66 ± 0.88 |
10.33 ± 3.18 |
|
2nd degree, % |
12.33 ± 1.85 |
18.66 ± 1.67 |
19.33 ± 2.33 |
|
3rd degree, % |
21.33 ± 3.53 |
42.66 ± 1.33** |
43.00 ± 3.21* |
|
Average cytochemical coefficient, conditional units |
0.98 ± 0.08 |
1.75 ± 0.06** |
1.78 ± 0.02** |
|
Day 28 after inoculation of Mycobacterium (day 14 after administration of the preparation) |
|||
|
1st degree, % |
5.66 ± 0.67 |
15.00 ± 1.53 |
10.00 ± 0.58** |
|
2nd degree, % |
7.33 ± 2.60 |
14.66 ± 2.33 |
13.00 ± 2.08 |
|
3rd degree, % |
23.33 ± 0.88 |
36.00 ± 5.68 |
44.66 ± 4.98* |
|
Average cytochemical coefficient, conditional units |
0.90 ± 0.06 |
1.52 ± 0.13* |
1.70 ± 0.18* |
|
Day 42 after inoculation of Mycobacterium (day 28 after administration of the preparation) |
|||
|
1st degree, % |
7.66 ± 1.33 |
7.33 ± 0.33 |
5.66 ± 1.85 |
|
2nd degree, % |
8.66 ± 2.33 |
13.66 ± 3.18 |
12.66 ± 1.45 |
|
3rd degree, % |
26.00 ± 1.00 |
23.33 ± 2.03 |
59.33 ± 0.88*** |
|
Average cytochemical coefficient, conditional units |
1.03 ± 0.03 |
1.05 ± 0.04 |
2.09 ± 0.01*** |
*p < 0.05; **p < 0.01; ***p < 0.001.
Later, significantly increased myeloperoxidase enzyme activity was observed in guinea pigs of experimental group 2. Thus, the average cytochemical coefficients in the group after administration of the preparation were:
– on day 14, 1.70 ± 0.18 c. u. versus 0.90 ± 0.06 c. u. (p < 0.05) in the control;
– on day 28, 2.09 ± 0.01 c. u. versus 1.03 ± 0.03 c. u. (p < 0.001) in the control.
In contrast, as the time after inoculation with mycobacteria passed by, experimental group 1 demonstrated a decrease in the oxygen-dependent metabolism of neutrophils to the level of the control group (by day 42 from the beginning of the experiment).
Indirect immunofluorescence of blood samples tested on day 42 after inoculation of Mycobacterium scrofulaceum, demonstrated mycobacterial antigen only in 2 guinea pigs from experimental group 1.
Thus, administration of the immunobiological product enhances functional activity of aerobic and anaerobic neutrophil bactericidal systems resulting in accelerated elimination of non-tuberculous mycobacteria from the experimental animals.
Histopathological tests conducted on day 45 from the start of the experiment also demonstrate reduced antigen load on the guinea pigs treated with the experimental conjugate. Thus, an increase in the number of lymphatic follicles with a large proliferation center was observed in the regional inguinal lymph nodes of the animals from experimental group 1 (Fig. 1), where macrophage hyperplasia was recorded. Macrophage proliferation was also found in the cortex. The medullary cords housed mainly lymphocytes and an insignificant number of plasmocytes.
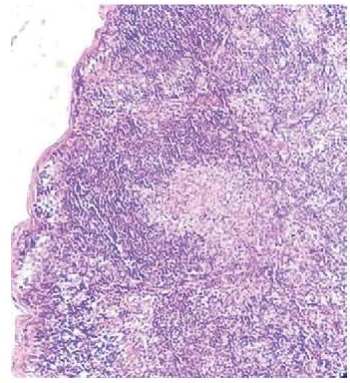
Fig. 1. A lymphoid follicle with a large germinal center.
Regional lymph node of a guinea pig (group 1).
Staining with hematoxylin and eosin, magnification 50×
In contrast, inguinal lymph node cortex in experimental group 2 was significantly thinner. The lymphoid follicles were also smaller; moreover, they lacked proliferation centers (Fig. 2), even if they had such centers, there were only dendritic reticulocytes in them.
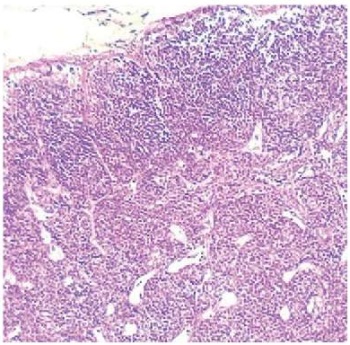
Fig. 2. Reduction of cortical substance volume
and size of lymphatic follicles without germinal centres.
Regional lymph node of a guinea pig (group 2).
Staining with hematoxylin and eosin, magnification 50×
As for the inguinal lymph nodes adjacent to the site of Mycobacterium scrofulaceum inoculation, significantly fewer lymph follicles were observed there compared with the regional lymph nodes in the same group. Fewer proliferation centres were observed in them, and fewer macrophages were found in the proliferation centres and stroma. The animals treated with the preparation (group 2) had even fewer lymph follicles in the cortex of the lymph nodes located opposite to the regional ones.
CONCLUSION
The performed experiments demonstrate that sensitization of guinea pigs with Mycobacterium scrofulaceum induces hyper-reactivity of neutrophil intracellular bactericidal components lasting up to 28 days. Further on, there is a drop in their activity to the level recorded in animals of the control group. Administration of the experimental preparation accelerates withdrawal of mycobacteria from the guinea pigs (on day 7 post administration) owing to stimulation of phagocytes, which is confirmed by immunofluorescence and histological tests.
Contribution: Koshkin I. N. – conducting experiments, selection of scientific literature, preparation of digital images of microscopic tests, making article design; Vlasenko V. S. – concept of presentation, compilation of tables, statistical processing of results, interpretation of data and summarizing test results; Dengis N. A. – conducting experiments, assistance in the article design.
Вклад авторов: Кошкин И. Н. – проведение экспериментов, подбор научной литературы, подготовка цифровых снимков микроскопических исследований, оформление статьи; Власенко В. С. – концепция представления материалов, составление таблиц, статистическая обработка результатов, интерпретация данных и обобщение результатов исследования; Денгис Н. А. – проведение экспериментов, помощь в оформлении статьи.
References
1. Parte A. C., Sardà Carbasse J., Meier-Kolthoff J. P., Reimer L. C., Göker M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. International Journal of Systematic and Evolutionary Microbiology. 2020; 70 (11): 5607–5612. https://doi.org/10.1099/ijsem.0.004332
2. Ghielmetti G., Friedel U., Scherrer S., Sarno E., Landolt P., Dietz O., et al. Non-tuberculous Mycobacteria isolated from lymph nodes and faecal samples of healthy slaughtered cattle and the abattoir environment. Transboundary and Emerging Diseases. 2018; 65 (3): 711–718. https://doi.org/10.1111/tbed.12793
3. Naimanov A. H., Gulukin M. I., Tolstenko N. G., Vangeli E. P., Kalmykov V. M. Organization of the fight against animal tuberculosis in Russia. Veterinariya. 2019; (4): 3–7. https://doi.org/10.30896/0042-4846.2019.22.4.03-07 (in Russ.)
4. Baratov M. O., Sakidibirov O. P., Abduragimova R. M., Dzhabarova G. A. Immune and protective properties of non-tuberculosis acid-resistant mycobacteria. Problems of Development of the Agro-Industrial Complex of the Region. 2022; (1): 73–79. https://doi.org/10.52671/20790996_2022_1_73 (in Russ.)
5. Nuru A., Zewude A., Mohammed T., Wondale B., Teshome L., Getahun M., et al. Nontuberculosis mycobacteria are the major causes of tuberculosis like lesions in cattle slaughtered at Bahir Dar Abattoir, northwestern Ethiopia. BMC Veterinary Research. 2017; 13 (1):237. https://doi.org/10.1186/s12917-017-1168-3
6. Hernández-Jarguín A. M., Martínez-Burnes J., Molina-Salinas G. M., de la Cruz-Hernández N. I., Palomares-Rangel J. L., López Mayagoitia A., Barrios-García H. B. Isolation and histopathological changes associated with non-tuberculous mycobacteria in lymph nodes condemned at a bovine slaughterhouse. Veterinary Sciences. 2020; 7 (4):172. https://doi.org/10.3390/vetsci7040172
7. Gomez-Buendia A., Alvarez J., Bezos J., Mourelo J., Amado J., Saez J. L., et al. Non-tuberculous mycobacteria: occurrence in skin test cattle reactors from official tuberculosis-free herds. Frontiers in Veterinary Science. 2024; 11:1361788. https://doi.org/10.3389/fvets.2024.1361788
8. Kamalieva Yu. R., Mingaleev D. N., Ravilov R. Kh. Identification of non-tuberculosis mycobacteria isolated from cattle in the Republic of Tatarstan. Agrarian Science. 2021; 354 (11–12): 32–35. https://doi.org/10.32634/0869-8155-2021-354-11-12-32-35 (in Russ.)
9. Baratov M. O., Huseynova P. S. Actual bovine tuberculosis situation in the Republic of Dagestan. Veterinary Science Today. 2022; 11 (3): 222–228. https://doi.org/10.29326/2304-196X-2022-11-3-222-228
10. Biet F., Boschiroli M. L. Non-tuberculous mycobacterial infections of veterinary relevance. Research in Veterinary Science. 2014; 97 (Suppl.): S69–S77. https://doi.org/10.1016/j.rvsc.2014.08.007
11. Lara G. H. B., Ribeiro M. G., Leite C. Q. F., Paes A. C., Guazzelli A., da Silva A.V., et al. Occurrence of Mycobacteriumspp. and other pathogens in lymph nodes of slaughtered swine and wild boars (Sus scrofa). Research in Veterinary Science. 2011; 90 (2): 185–188. https://doi.org/10.1016/j.rvsc.2010.06.009
12. Klanicova-Zalewska B., Slana I. Presence and persistence of Mycobacterium avium and other nontuberculous mycobacteria in animal tissues and derived foods: a review. Meat Science. 2014; 98 (4): 835–841. https://doi.org/10.1016/j.meatsci.2014.08.001
13. Varela-Castro L., Barral M., Arnal M. C., Fernández de Luco D., Gortázar C., Garrido J. M., Sevilla I. A. Beyond tuberculosis: Diversity and implications of non-tuberculous mycobacteria at the wildlife-livestock interface. Transboundary and Emerging Diseases. 2022; 69 (5): e2978–e2993. https://doi.org/10.1111/tbed.14649
14. Muwonge A., Oloya J., Kankya C., Nielsen S., Godfroid J., Skjerve E., et al. Molecular characterization of Mycobacterium avium subspecies hominissuis isolated from humans, cattle and pigs in the Uganda cattle corridor using VNTR analysis. Infection, Genetics and Evolution. 2014; 21: 184–191. https://doi.org/10.1016/j.meegid.2013.11.012
15. Leão C., Canto A., Machado D., Sanches I. S., Couto I., Viveiros M., et al. Relatedness of Mycobacterium avium subspecies hominissuis clinical isolates of human and porcine origins assessed by MLVA. Veterinary Microbiology. 2014; 173 (1–2): 92–100. https://doi.org/10.1016/j.vetmic.2014.06.027
16. Kontturi A., Soini H., Ollgren J., Salo E. Increase in childhood nontuberculous mycobacterial infections after bacille Calmette-Guérin coverage drop: A nationwide, population-based retrospective study, Finland, 1995–2016. Clinical Infectious Diseases. 2018; 67 (8): 1256–1261. https://doi.org/10.1093/cid/ciy241
17. Zimmermann P., Finn A., Curtis N. Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis. The Journal of Infectious Diseases. 2018; 218 (5): 679–687. https://doi.org/10.1093/infdis/jiy207
18. Abate G., Hamzabegovic F., Eickhoff C. S., Hoft D. F. BCG vaccination induces M. avium and M. abscessus cross-protective immunity. Frontiers in Immunology. 2019; 10:234. https://doi.org/10.3389/fimmu.2019.00234
19. Fritschi N., Curtis N., Ritz N. Bacille Calmette Guérin (BCG) and new TB vaccines: Specific, cross-mycobacterial and off-target effects. Paediatric Respiratory Reviews. 2020; 36: 57–64. https://doi.org/10.1016/j.prrv.2020.08.004
20. Vlasenko V. S., Kosobokov E. A., Dengis N. A., Novikova N. N. Studying immunotherapeutic properties of the immunomodulator KIM-M2 in guinea pigs infected with nontuberculous mycobacteria. Bulletin of KrasSAU. 2022; (5): 91–97. https://doi.org/10.36718/1819-4036-2022-5-91-97 (in Russ.)
21. Orme I. M., Collins F. M. Efficacy of Mycobacterium bovis BCG vaccination in mice undergoing prior pulmonary infection with atypical mycobacteria. Infection and Immunity. 1984; 44 (1): 28–32. https://doi.org/10.1128/iai.44.1.28-32.1984
22. Buddle B. M., Wards B. J., Aldwell F. E., Collins D. M., de Lisle G. W. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine. 2002; 20 (7–8): 1126–1133. https://doi.org/10.1016/S0264-410X(01)00436-4
23. Palmer M. V., Thacker T. C. Use of the human vaccine, Mycobacterium bovis Bacillus Calmette Guérin in deer. Frontiers in Veterinary Science. 2018; 5:244. https://doi.org/10.3389/fvets.2018.00244
24. Shah J. A., Lindestam Arlehamn C. S., Horne D. J., Sette A., Hawn T. R. Nontuberculous mycobacteria and heterologous immunity to tuberculosis. The Journal of Infectious Diseases. 2019; 220 (7): 1091–1098. https://doi.org/10.1093/infdis/jiz285
25. Koshkin I. N., Vlasenko V. S., Pleshakova V. I., Alkhimova L. E., Elyshev A. V., Kulakov I. V. Morphology of lymphoid tissue in the lungs of guinea pigs infected with Mycobacterium bovis against the background of vaccine immunity and the action of betulin and its derivatives. Vaccines. 2022; 10 (12):2084. https://doi.org/10.3390/vaccines10122084
26. Novikova N. N., Baiseitov S. T., Vlasenko V. S., Krasikov A. P. Using indirect immunofluorescence to diagnose bovine leukosis: guidelines. Almaty: NOVA Press; 2020. 17 p. (in Russ.)
About the Authors
I. N. KoshkinRussian Federation
Ivan N. Koshkin, Cand. Sci. (Veterinary Medicine), Senior Researcher, Laboratory of Epizootology and Tuberculosis Control, Department of Veterinary Medicine
26 Koroleva ave., Omsk 644012
V. S. Vlasenko
Russian Federation
Vasily S. Vlasenko, Dr. Sci. (Biology), Professor, Chief Researcher, Laboratory of Epizootology and Tuberculosis Control, Department of Veterinary Medicine
26 Koroleva ave., Omsk 644012
N. A. Dengis
Russian Federation
Natalia A. Dengis, Cand. Sci. (Biology), Leading Researcher, Laboratory of Epizootology and Tuberculosis Control, Department of Veterinary Medicine
26 Koroleva ave., Omsk 644012
Review
For citations:
Koshkin I.N., Vlasenko V.S., Dengis N.A. Studying immunotherapeutic properties of the conjugate based on BCG antigens with betulonic acid in guinea pigs infected with Mycobacterium scrofulaceum. Veterinary Science Today. 2024;13(2):183-188. https://doi.org/10.29326/2304-196X-2024-13-2-183-188



































